What is the most common form of nitrogen found in the atmosphere?
Nitrate
Nitrite
Ammonia
Nitrogen gas
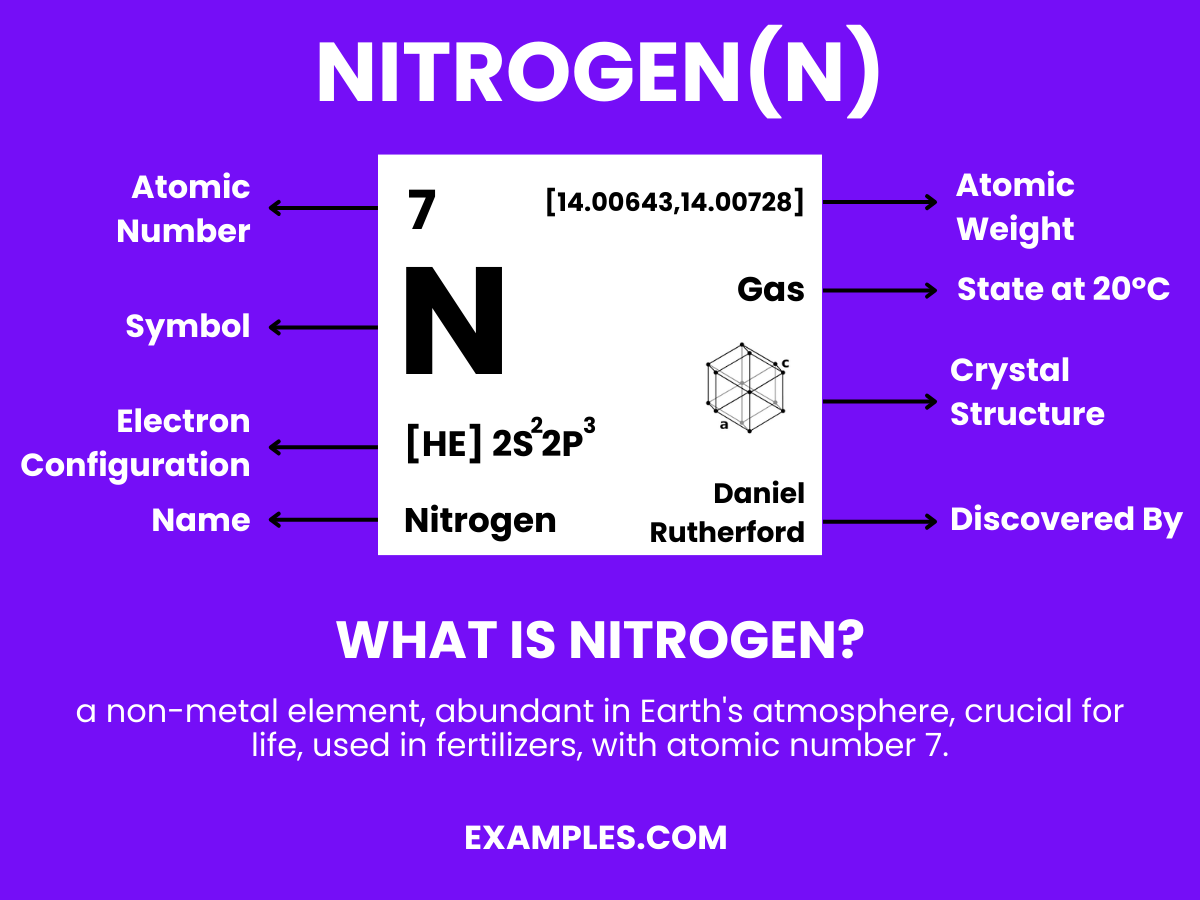
Discover Nitrogen, a fundamental element that pairs with hydrogen to form the building blocks of life. This guide takes you through the journey of Nitrogen’s role in the atmosphere, its usage in various industries, and its presence in organic compounds. Understanding Nitrogen is key in fields ranging from agriculture to medicine. Dive into real-world examples, explore its dynamic behaviors, and grasp its essentiality in forming amino acids, DNA, and the air we breathe.
Nitrogen is a colorless, odorless, tasteless gas that constitutes about 78% of the Earth’s atmosphere by volume. Represented by the symbol ‘N’ and atomic number 7, it is a nonmetal with five electrons in its outer shell. Nitrogen is crucial for all living organisms as it is a key component of amino acids, proteins, and nucleic acids (DNA & RNA). It exists primarily as diatomic molecules (N₂), making it inert and stable under standard conditions. In the vast realm of chemistry, Nitrogen plays a pivotal role in creating a diverse range of compounds, from the simplicity of ammonia (NH₃) to the complexity of organic molecules, and is essential in both the industrial and biological spheres.
| Hydrogen | Sulfur |
| Carbon | Chlorine |
| Oxygen | Selenium |
| Fluorine | Bromine |
| Phosphorus | Iodine |
| Property | Description |
|---|---|
| Symbol | N |
| Atomic Number | 7 |
| Atomic Mass | 14.007 amu |
| State at Room Temperature | Gas |
| Color | Colorless |
| Electronegativity (Pauling Scale) | 3.04 |
| Ionization Energies | 1st: 1402.3 kJ/mol |
| Atomic Radius | 56 pm |
| Electron Configuration | 1s² 2s² 2p³ |
| Oxidation States | -3, -2, -1, +1, +2, +3, +4, +5 |
| Isotopes | Most stable are Nitrogen-14 and Nitrogen-15 |
| Reactivity | Generally inert due to strong triple bond in N₂, reactive under certain conditions |
| Compounds | Forms a variety of compounds like ammonia (NH₃), nitric acid (HNO₃), nitrates (NO₃⁻), and nitrites (NO₂⁻) |
| Melting Point | -210.00 °C |
| Boiling Point | -195.79 °C |
| Density | 0.0012506 grams/cm³ (at 0°C and 1 atm) |
| Solubility in Water | Poor, but soluble under high pressure and low temperature |
| Property | Value with Unit |
|---|---|
| Boiling Point | -195.79 °C |
| Melting Point | -210.00 °C |
| Critical Temperature | -147.1 °C |
| Critical Pressure | 3.39 MPa |
| Heat of Vaporization | 5.56 kJ/mol |
| Heat of Fusion | 0.72 kJ/mol |
| Specific Heat Capacity (at 15°C) | 1.04 J/g·K |
| Thermal Conductivity | 0.02583 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 0°C and 1 atm) | 1.2506 kg/m³ |
| Viscosity (at -195.79°C) | 0.000158 Pa·s |
| Solubility in Water (at 0°C) | 0.0235 g/100 mL of water |
| Refractive Index (at -183°C) | 1.000297 |
| Surface Tension (at -195.79°C) | 8.85 mN/m |
| Property | Value with Unit |
|---|---|
| Electric Dipole Moment | 0 Debye |
| Ionization Energy | 14.534 eV |
| Electron Affinity | -6.8 kJ/mol |
| Electrical Conductivity | Non-conductive |
| Magnetic Susceptibility | -12.0 × 10^-6 cm^3/mol |
| Property | Value with Unit |
|---|---|
| Atomic Number | 7 |
| Atomic Mass | 14.007 amu |
| Isotopes | ¹⁴N (99.63%), ¹⁵N (0.37%) |
| Nuclear Spin (for ¹⁴N) | 1 ℏ |
| Nuclear Spin (for ¹⁵N) | 1/2 ℏ |
| Neutron Cross Section (for ¹⁴N) | 1.83 barns |
| Neutron Cross Section (for ¹⁵N) | 0.000024 barns |
| Nuclear Magnetic Moment (for ¹⁴N) | 0.40376100 µN |
| Nuclear Magnetic Moment (for ¹⁵N) | -0.28318884 µN |
The Nitrogen Cycle is a biogeochemical process that illustrates the movement and transformation of nitrogen through various phases in the environment, including the atmosphere, lithosphere, hydrosphere, and biosphere. Here’s a simplified breakdown of the nitrogen cycle stages:
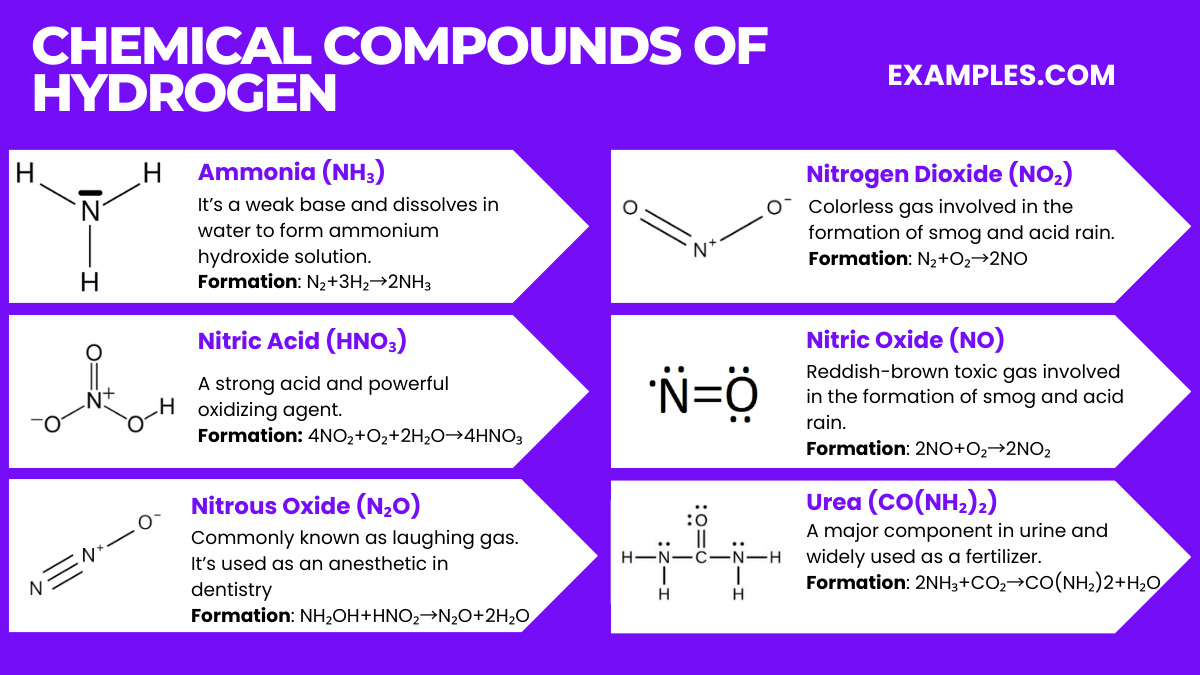
Nitrogen, a versatile and widely abundant element, forms a variety of chemical compounds that are essential to life and industry. Understanding these compounds provides insight into environmental processes, industrial applications, and biological functions. Here are some key nitrogen compounds:
| Isotope | Symbol | Neutrons | Protons | Natural Abundance | Properties |
|---|---|---|---|---|---|
| Nitrogen-14 | ¹⁴N | 7 | 7 | Approx. 99.63% | Stable, non-radioactive, most common isotope |
| Nitrogen-15 | ¹⁵N | 8 | 7 | Approx. 0.37% | Stable, non-radioactive, used as a tracer in scientific research |
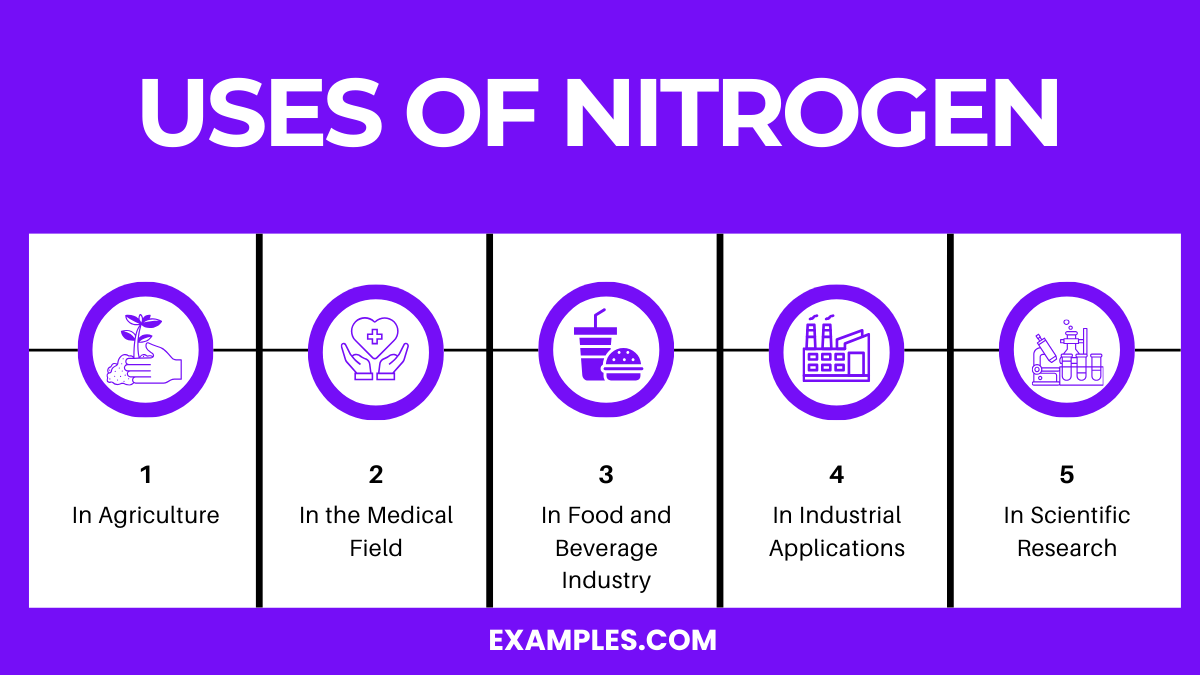
Nitrogen, a versatile and widely abundant element, is crucial in various sectors due to its unique properties. It’s the most abundant gas in Earth’s atmosphere and has numerous applications in the industrial, technological, and scientific sectors. Understanding its uses is pivotal for educators and students alike to appreciate this element’s versatility.
Nitrogen is one of the most abundantly available elements on earth, making up about 78% of the Earth’s atmosphere. Its vast availability makes it essential for various commercial and industrial applications. Here is a comprehensive guide to the commercial production of nitrogen, aimed at providing educators with detailed insights into the process.
Educators can utilize this comprehensive guide to enlighten students about the critical role nitrogen plays in everyday life, from the food we eat to the technologies we depend on. Understanding nitrogen’s uses not only conveys its importance in various industries but also instills a deeper appreciation for this fundamental element in the natural world.
Nitrogen, as an elemental gas in its diatomic form (N₂), is relatively inert and non-toxic, making up about 78% of the Earth’s atmosphere. However, its various compounds and forms can have different health effects, ranging from beneficial to harmful. Here are some of the health effects associated with nitrogen and its compounds:
Despite some of the hazards associated with nitrogen compounds, nitrogen also has numerous benefits and is essential for life:
Proteins, DNA, and chlorophyll are made up of nitrogen, essential for all living organisms’ structure and metabolic processes.
Nitrogen is used in fertilizers, food preservation, industrial applications, and medical procedures such as cryopreservation.
In humans, nitrogen is crucial for building proteins, nucleic acids, and is a significant component of our genetic material.
Nitrogen is a gas, making up about 78% of Earth’s atmosphere by volume; it’s colorless, odorless, and mostly inert.
Nitrogen is essential and non-toxic in its molecular form (N₂), but certain nitrogen compounds or excessive amounts can be harmful.
Nitrogen is a fundamental element that permeates life, industry, and the environment. Understanding its versatile roles, from its essential presence in organic compounds to its vast industrial applications, can enhance both educational and practical approaches to science. With this guide, embrace nitrogen’s complexities and its vital contributions to our world. Use this knowledge responsibly and innovatively for a sustainable future.

Discover Nitrogen, a fundamental element that pairs with hydrogen to form the building blocks of life. This guide takes you through the journey of Nitrogen’s role in the atmosphere, its usage in various industries, and its presence in organic compounds. Understanding Nitrogen is key in fields ranging from agriculture to medicine. Dive into real-world examples, explore its dynamic behaviors, and grasp its essentiality in forming amino acids, DNA, and the air we breathe.

Nitrogen is a colorless, odorless, tasteless gas that constitutes about 78% of the Earth’s atmosphere by volume. Represented by the symbol ‘N’ and atomic number 7, it is a nonmetal with five electrons in its outer shell. Nitrogen is crucial for all living organisms as it is a key component of amino acids, proteins, and nucleic acids (DNA & RNA). It exists primarily as diatomic molecules (N₂), making it inert and stable under standard conditions. In the vast realm of chemistry, Nitrogen plays a pivotal role in creating a diverse range of compounds, from the simplicity of ammonia (NH₃) to the complexity of organic molecules, and is essential in both the industrial and biological spheres.
Formula: N₂
Composition: Consists of two nitrogen atoms.
Bond Type: The two nitrogen atoms are connected by a strong triple bond.
Molecular Structure: Diatomic, meaning it contains two atoms forming a N≡N bond.
Electron Configuration: Each nitrogen atom has five valence electrons, with a total of ten electrons when in N₂ form.
Significance: Nitrogen is essential to life, making up a fundamental component of amino acids, proteins, and nucleic acids. It also makes up about 78% of Earth’s atmosphere.
Role in Chemistry: Nitrogen’s reactivity and various oxidation states allow it to form a wide range of compounds, from simple molecules like ammonia (NH₃) to complex organic structures. It is also used industrially in the production of fertilizers, nitric acid, nylon, dyes, and explosives.

Molecular Composition: Nitrogen gas consists of two nitrogen atoms.
Atomic Arrangement: In a nitrogen molecule (N₂), the two nitrogen atoms are joined by a strong triple covalent bond.
Bonding: The triple bond between the two nitrogen atoms in N₂ includes one sigma bond and two pi bonds. This bonding arrangement contributes to the molecule’s stability and relative inertness under normal conditions.
Molecular Geometry: The molecule is linear, with a bond angle of 180 degrees.
Electron Configuration: Each nitrogen atom in the molecule has five valence electrons, making a total of ten electrons that participate in bonding and non-bonding interactions.

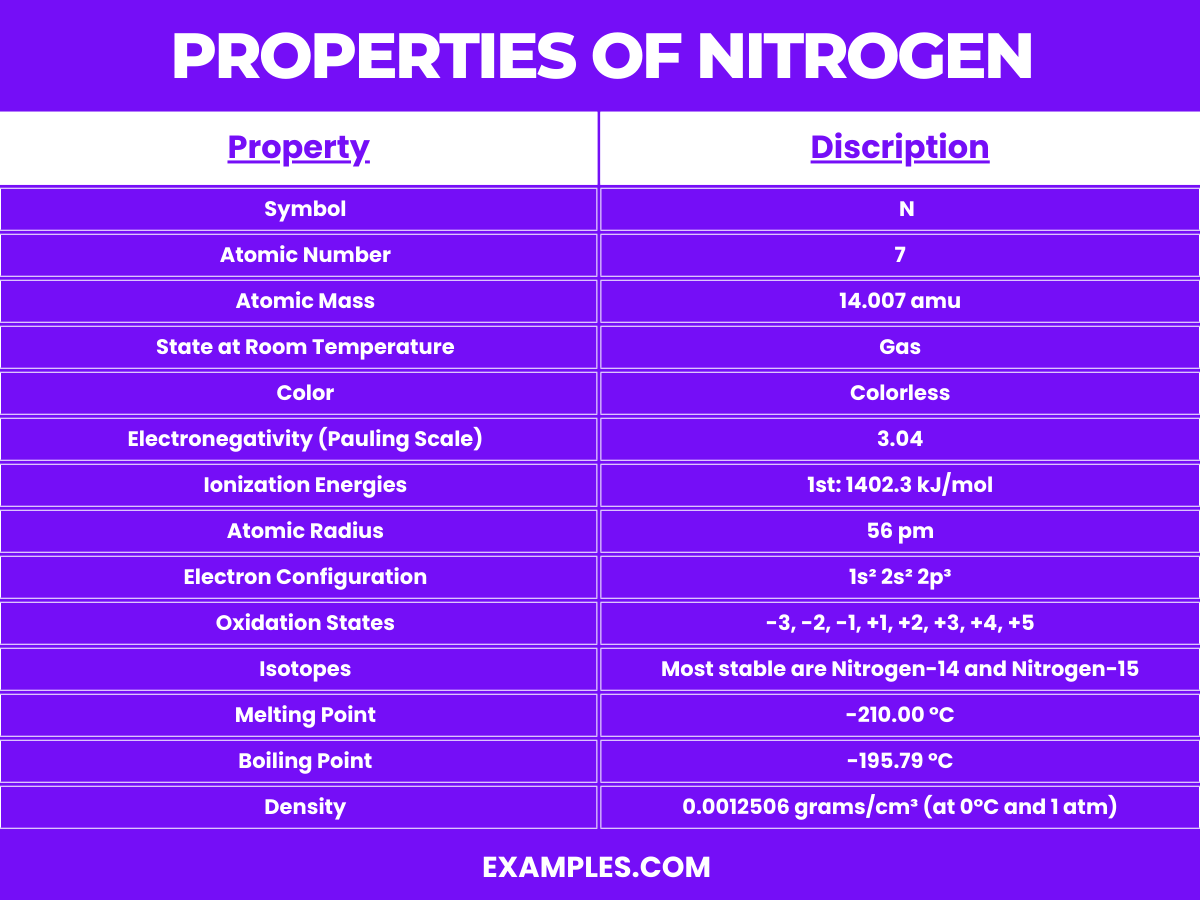
Property | Description |
|---|---|
Symbol | N |
Atomic Number | 7 |
Atomic Mass | 14.007 amu |
State at Room Temperature | Gas |
Color | Colorless |
Electronegativity (Pauling Scale) | 3.04 |
Ionization Energies | 1st: 1402.3 kJ/mol |
Atomic Radius | 56 pm |
Electron Configuration | 1s² 2s² 2p³ |
Oxidation States | -3, -2, -1, +1, +2, +3, +4, +5 |
Isotopes | Most stable are Nitrogen-14 and Nitrogen-15 |
Reactivity | Generally inert due to strong triple bond in N₂, reactive under certain conditions |
Compounds | Forms a variety of compounds like ammonia (NH₃), nitric acid (HNO₃), nitrates (NO₃⁻), and nitrites (NO₂⁻) |
Melting Point | -210.00 °C |
Boiling Point | -195.79 °C |
Density | 0.0012506 grams/cm³ (at 0°C and 1 atm) |
Solubility in Water | Poor, but soluble under high pressure and low temperature |
Property | Value with Unit |
|---|---|
Boiling Point | -195.79 °C |
Melting Point | -210.00 °C |
Critical Temperature | -147.1 °C |
Critical Pressure | 3.39 MPa |
Heat of Vaporization | 5.56 kJ/mol |
Heat of Fusion | 0.72 kJ/mol |
Specific Heat Capacity (at 15°C) | 1.04 J/g·K |
Thermal Conductivity | 0.02583 W/m·K |
Property | Value with Unit |
|---|---|
Density (at 0°C and 1 atm) | 1.2506 kg/m³ |
Viscosity (at -195.79°C) | 0.000158 Pa·s |
Solubility in Water (at 0°C) | 0.0235 g/100 mL of water |
Refractive Index (at -183°C) | 1.000297 |
Surface Tension (at -195.79°C) | 8.85 mN/m |
Property | Value with Unit |
|---|---|
Electric Dipole Moment | 0 Debye |
Ionization Energy | 14.534 eV |
Electron Affinity | -6.8 kJ/mol |
Electrical Conductivity | Non-conductive |
Magnetic Susceptibility | -12.0 × 10^-6 cm^3/mol |
Property | Value with Unit |
|---|---|
Atomic Number | 7 |
Atomic Mass | 14.007 amu |
Isotopes | ¹⁴N (99.63%), ¹⁵N (0.37%) |
Nuclear Spin (for ¹⁴N) | 1 ℏ |
Nuclear Spin (for ¹⁵N) | 1/2 ℏ |
Neutron Cross Section (for ¹⁴N) | 1.83 barns |
Neutron Cross Section (for ¹⁵N) | 0.000024 barns |
Nuclear Magnetic Moment (for ¹⁴N) | 0.40376100 µN |
Nuclear Magnetic Moment (for ¹⁵N) | -0.28318884 µN |
The Nitrogen Cycle is a biogeochemical process that illustrates the movement and transformation of nitrogen through various phases in the environment, including the atmosphere, lithosphere, hydrosphere, and biosphere. Here’s a simplified breakdown of the nitrogen cycle stages:
Description: Conversion of atmospheric nitrogen (N₂) into ammonia (NH₃) or related compounds, making nitrogen available to living organisms.
Biological Fixation: Performed by certain bacteria and cyanobacteria using the enzyme nitrogenase.
Equation: N₂+8H⁺+8e⁻→2NH₃+H₂
Industrial Fixation: Achieved through the Haber-Bosch process for creating fertilizers.
Equation: N₂+3H₂→2NH₃
Description: The conversion of ammonia into nitrites (NO₂⁻) and then nitrates (NO₃⁻) by nitrifying bacteria.
Steps:
Ammonia to Nitrite: Carried out by bacteria like Nitrosomonas.
Equation: 2NH₃+3O₂→2NO₂⁻+2H⁺+2H₂O
Nitrite to Nitrate: Conducted by bacteria such as Nitrobacter.
Equation: 2NO₂⁻+O₂→2NO₃⁻
Description: Plants absorb nitrates and ammonium ions from the soil, incorporating them into plant proteins and nucleic acids.
Process: Nitrates are reduced to nitrites and then ammonia before being incorporated into organic molecules.
Equation: Varied, depending on the organic compound being synthesized.
Description: The conversion of organic nitrogen back into ammonia (NH₃) or ammonium ions (NH₄⁺) by decomposing bacteria and fungi.
Process: Breakdown of dead organisms and other organic waste materials.
Equation: organic−N→NH₃/NH₄⁺
Description: The reduction of nitrates back into the largely inert nitrogen gas (N₂), completing the cycle.
Process: Carried out by denitrifying bacteria (e.g., Pseudomonas and Clostridium) under anaerobic conditions.
Equation: 2NO₃⁻+10e⁻+12H+→N₂+6H₂O

Nitrogen, a versatile and widely abundant element, forms a variety of chemical compounds that are essential to life and industry. Understanding these compounds provides insight into environmental processes, industrial applications, and biological functions. Here are some key nitrogen compounds:
Formation: N₂+3H₂→2NH₃ (Haber process)
Properties: Ammonia is a colorless gas with a pungent smell. It’s a weak base and dissolves in water to form ammonium hydroxide solution. It’s used in fertilizers, cleaning products, and as a refrigerant.
Formation: 4NO₂+O₂+2H₂O→4HNO₃
Properties: A strong acid and powerful oxidizing agent. It’s used in fertilizers, explosives, and many industrial processes.
Formation: NH₂OH+HNO₂→N₂O+2H₂O (One of several methods)
Properties: Commonly known as laughing gas. It’s used as an anesthetic in dentistry and as a propellant in food packaging.
Formation: N₂+O₂→2NO followed by 2NO+O₂→2NO₂
Properties: NO is a colorless gas, while NO₂ is a reddish-brown toxic gas. Both are significant pollutants and are involved in the formation of smog and acid rain.
Formation: 2NH₃+CO₂→CO(NH₂)2+H₂O
Properties: A major component in urine and widely used as a nitrogen-release fertilizer. It’s also used in plastics, animal feed, and pharmaceuticals.
Isotope | Symbol | Neutrons | Protons | Natural Abundance | Properties |
|---|---|---|---|---|---|
Nitrogen-14 | ¹⁴N | 7 | 7 | Approx. 99.63% | Stable, non-radioactive, most common isotope |
Nitrogen-15 | ¹⁵N | 8 | 7 | Approx. 0.37% | Stable, non-radioactive, used as a tracer in scientific research |

Nitrogen, a versatile and widely abundant element, is crucial in various sectors due to its unique properties. It’s the most abundant gas in Earth’s atmosphere and has numerous applications in the industrial, technological, and scientific sectors. Understanding its uses is pivotal for educators and students alike to appreciate this element’s versatility.
Fertilizers: Nitrogen is a key component of amino acids, the building blocks of proteins, and is crucial for plant growth. Ammonia (NH₃), nitrates (NO₃⁻), and urea (CO(NH₂)₂) are widely used nitrogenous fertilizers.
Soil Nutrient: Nitrogen-fixing bacteria in the soil convert atmospheric nitrogen (N₂) into forms that plants can absorb and utilize.
Cryopreservation: Liquid nitrogen is used for the cryopreservation of biological samples, including blood, sperm, eggs, and other cell types.
Medical Devices: Nitrogen gas is utilized in medical devices and as a propellant in aerosolized medications.
Food Preservation: Nitrogen gas is used in food packaging to prevent oxidation and spoilage, maintaining freshness and extending shelf life.
Beverage Industry: It’s used in carbonated beverages and to push beer from kegs to taps.
Manufacturing and Construction: As an inert gas, nitrogen is used to prevent oxidation in metal processing, welding, and soldering.
Electronics: Nitrogen is used in the production of transistors, diodes, and integrated circuits.
Chemical and Pharmaceutical Research: Nitrogen compounds are fundamental in developing a wide array of industrial chemicals and pharmaceuticals.
Nitrogen is one of the most abundantly available elements on earth, making up about 78% of the Earth’s atmosphere. Its vast availability makes it essential for various commercial and industrial applications. Here is a comprehensive guide to the commercial production of nitrogen, aimed at providing educators with detailed insights into the process.
Process: The air is first purified by removing water vapor, carbon dioxide, and other impurities. It’s then compressed and cooled to extremely low temperatures, turning it into a liquid. This liquid air is then subjected to fractional distillation, separating nitrogen due to its different boiling point from oxygen and other gases.
Usage: This method produces high purity nitrogen and is widely used for industrial purposes where large volumes of nitrogen are required.
Process: PSA technology uses adsorbent materials that preferentially adsorb oxygen over nitrogen from the air at high pressure. When the pressure is released, the adsorbed gases are desorbed, leaving nearly pure nitrogen.
Usage: This method is commonly used for on-site nitrogen generation and provides a continuous supply of nitrogen with adjustable purity levels.
Process: Air is passed through a semi-permeable membrane that selectively allows oxygen, water vapor, and other impurities to pass through, while nitrogen is retained on the other side.
Usage: This method is suitable for applications requiring lower purity nitrogen and offers the benefits of compact equipment and energy efficiency.
Ammonia Synthesis: The Haber process combines nitrogen from the air with hydrogen derived from natural gas (methane) to produce ammonia, a precursor to many nitrogen-containing compounds.
Nitric Acid Production: Ammonia is further oxidized to produce nitric acid, used in fertilizers and explosives.
Oil Recovery: Nitrogen is injected into oil wells to help force crude oil to the surface.
Refining Processes: It’s used to create an inert atmosphere to prevent explosions or fires.
Metal Treatment: Nitrogen is used in heat-treating processes to strengthen and harden metals.
Stainless Steel Manufacturing: It’s used as a shielding gas in the production of stainless steel.
Tire Inflation: Nitrogen is used to inflate airplane and race car tires due to its inertness and consistency under temperature fluctuations.
Modified Atmosphere Packaging: Nitrogen gas is used to displace oxygen in packaging, reducing oxidative damage and spoilage in foods.
Educators can utilize this comprehensive guide to enlighten students about the critical role nitrogen plays in everyday life, from the food we eat to the technologies we depend on. Understanding nitrogen’s uses not only conveys its importance in various industries but also instills a deeper appreciation for this fundamental element in the natural world.
Nitrogen, as an elemental gas in its diatomic form (N₂), is relatively inert and non-toxic, making up about 78% of the Earth’s atmosphere. However, its various compounds and forms can have different health effects, ranging from beneficial to harmful. Here are some of the health effects associated with nitrogen and its compounds:
Asphyxiation Risk: Although nitrogen is non-toxic, in high concentrations, it can displace oxygen in the air, leading to asphyxiation. This is a concern in enclosed spaces or industrial settings where nitrogen gas is used.
Respiratory Problems: Exposure to nitrogen oxides, which are produced during combustion processes, can irritate the lungs, leading to respiratory problems and exacerbating conditions like asthma.
Environmental Health: Nitrogen oxides contribute to smog and acid rain, which have broader health impacts on populations.
Toxicity: Ammonia, when inhaled or in contact with skin, can be caustic and toxic, causing irritation and in severe cases, chemical burns or lung damage.
Methemoglobinemia: Nitrates and nitrites can convert hemoglobin into methemoglobin, which is unable to carry oxygen effectively. This condition, known as methemoglobinemia, is particularly risky for infants.
Despite some of the hazards associated with nitrogen compounds, nitrogen also has numerous benefits and is essential for life:
Protein Synthesis: Nitrogen is a key component of amino acids, the building blocks of proteins, making it essential for growth, repair, and functionality of all living cells.
DNA Structure: It is also a component of nucleotides, which are the building blocks of DNA and RNA, crucial for genetic inheritance and cellular functions.
Cryopreservation: Liquid nitrogen is used to preserve blood, sperm, eggs, and other biological samples, a process critical for medical treatments and research.
Dermatology: Liquid nitrogen is used in cryotherapy to freeze and remove warts, precancerous cells, and other skin lesions.
Fertilizers: Nitrogen is a significant component of fertilizers, enhancing soil fertility and plant growth, which is crucial for global food production.
Inert Atmosphere: Nitrogen is used to create an inert atmosphere in various industrial processes, preventing oxidation or explosions.
Food Packaging: Nitrogen is used in food packaging to displace oxygen, extending shelf life and freshness.
Proteins, DNA, and chlorophyll are made up of nitrogen, essential for all living organisms’ structure and metabolic processes.
Nitrogen is used in fertilizers, food preservation, industrial applications, and medical procedures such as cryopreservation.
In humans, nitrogen is crucial for building proteins, nucleic acids, and is a significant component of our genetic material.
Nitrogen is a gas, making up about 78% of Earth’s atmosphere by volume; it’s colorless, odorless, and mostly inert.
Nitrogen is essential and non-toxic in its molecular form (N₂), but certain nitrogen compounds or excessive amounts can be harmful.
Nitrogen is a fundamental element that permeates life, industry, and the environment. Understanding its versatile roles, from its essential presence in organic compounds to its vast industrial applications, can enhance both educational and practical approaches to science. With this guide, embrace nitrogen’s complexities and its vital contributions to our world. Use this knowledge responsibly and innovatively for a sustainable future.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the most common form of nitrogen found in the atmosphere?
Nitrate
Nitrite
Ammonia
Nitrogen gas
Which of the following is a use of nitrogen in the industrial sector?
Ripening fruits
Welding and metal cutting
Water purification
Bleaching fabrics
What role does nitrogen play in the ecosystem?
Primary energy source
Helps in soil erosion
Nutrient for plant growth
Regulates water cycles
How do animals and humans primarily obtain nitrogen?
Breathing nitrogen gas
Consuming plants or plant-consuming animals
Drinking water
Exposure to sunlight
Which process converts atmospheric nitrogen into a usable form for plants?
Photosynthesis
Nitrification
Nitrogen fixation
Decomposition
Which compound results from the industrial fixation of nitrogen?
Nitric acid
Ammonia
Nitrogen gas
Nitrous oxide
What is a common agricultural problem associated with excess nitrogen?
Reduced crop yield
Soil acidification
Increased pest resistance
Enhanced flavor in crops
In which form is nitrogen most commonly absorbed by plants?
Nitrogen gas
Ammonium
Nitrate
Urea
What is the main environmental concern related to nitrogen-based fertilizers?
Air pollution
Water pollution
Noise pollution
Light pollution
Which enzyme is crucial for the process of nitrogen fixation in plants?
Amylase
Nitrogenase
Catalase
Rubisco
Before you leave, take our quick quiz to enhance your learning!

