What is the chemical symbol for Nobelium?
No
Nb
Nm
Ne
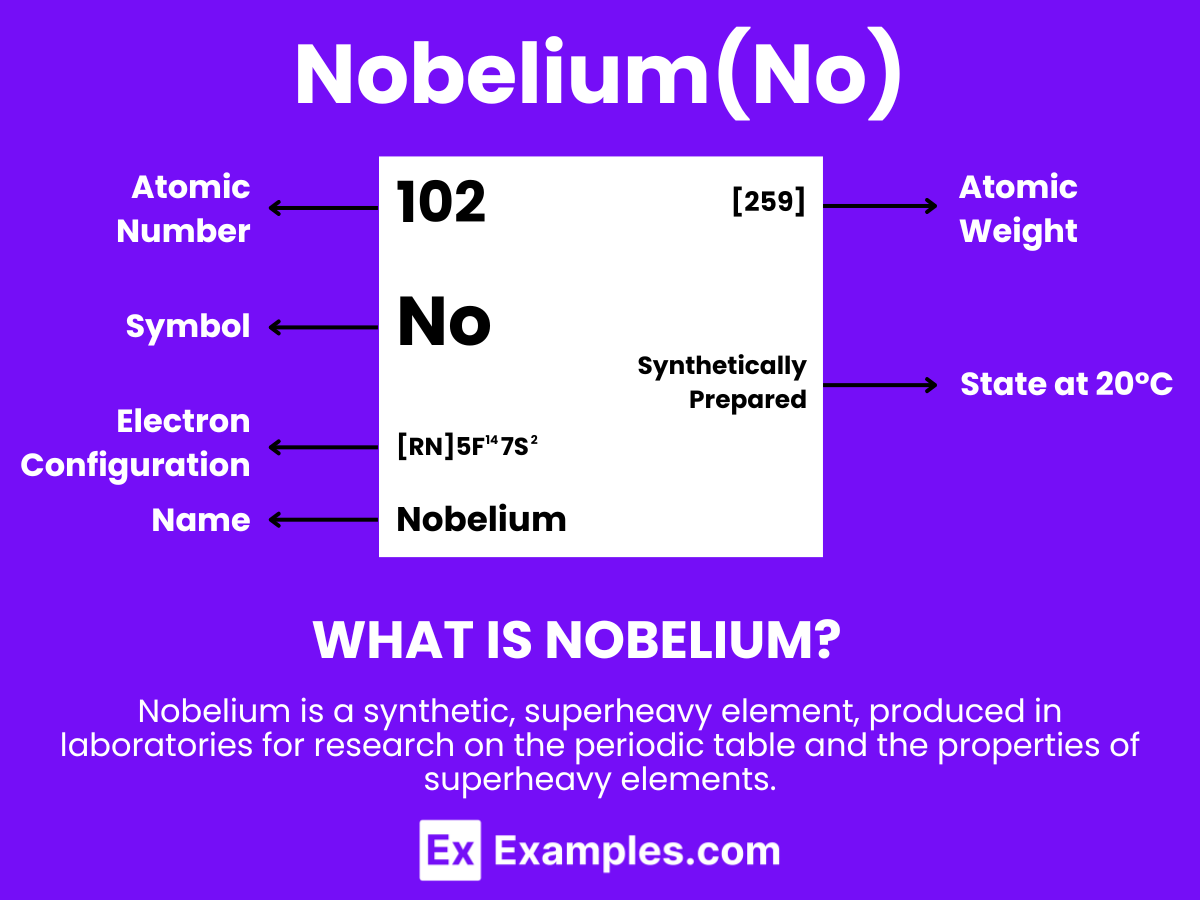
Embark on a scientific journey into the realm of Nobelium, a synthetic element with an air of mystery. Nobelium, atomic number 102, resides in the actinide series of the periodic table, where its fleeting existence challenges researchers to uncover its secrets. This transuranic heavyweight is synthesized in minute quantities, offering a playground for cutting-edge nuclear research and advanced spectroscopic studies. Understanding Nobelium’s properties and potential applications not only captivates chemists but also provides valuable insights into the architecture of the atomic nucleus. Dive deep into the intricacies of this enigmatic element, and discover how Nobelium continues to shape our grasp of synthetic elements in modern science.
Nobelium is a synthetic element with the chemical symbol No and atomic number 102. It is known for being produced in particle accelerators through the bombardment of atomic nuclei. Nobelium does not occur in nature and has a very short lifespan before it decays, which presents challenges for its study. The element’s discovery is crucial for nuclear physics research, especially in exploring the properties and behaviors of transuranium elements in the periodic table. Because of its significant instability and radioactivity, nobelium has no practical applications beyond scientific inquiry, where it contributes to our understanding of the chemical characteristics of heavy elements and the limits of the periodic table.
Understanding the atomic structure of Nobelium offers intriguing insights into its distinctive role within the periodic table and the field of nuclear chemistry. Possessing 102 protons in its nucleus, Nobelium is definitively categorized, setting it apart in terms of its chemical properties and its limited capacity for molecular formation.
Atomic Level: Each atom of Nobelium (No) is defined by the presence of 102 protons in its nucleus, which establishes its atomic number as 102. The theoretical electron configuration for Nobelium is [Rn]5f¹⁴ 7s², suggesting a complete 5f orbital and two electrons in the 7s orbital, which forms the basis for potential chemical interactions. However, the influence of relativistic effects is anticipated to significantly modify its actual electron configuration, which could, in turn, affect its chemical properties.
Molecular Formation: Nobelium, much like Mendelevium, does not naturally form stable molecules or exhibit a consistent molecular structure due to its incredibly short half-life and pronounced instability. This element exists momentarily before undergoing decay into lighter elements, rendering the exploration of its bonding nature and potential for molecular formation largely a theoretical pursuit. Should Nobelium atoms manage to exist long enough to chemically interact, their behavior is expected to be shaped by their electron configuration, although such scenarios are purely speculative.
The stability and phase of Nobelium under differing temperatures and pressures remain a matter of theoretical conjecture, as its transient nature makes it impossible to observe its state as solid, liquid, or gas under standard conditions.
| Property | Value |
|---|---|
| Appearance | Unknown; presumably metallic |
| Atomic Number | No |
| Atomic Mass | (259)amu |
| State at 20 °C | Solid (predicted) |
| Melting Point | 827 °C (predicted, though uncertainty exists) |
| Boiling Point | Not precisely known; estimates suggest it could be around 827 °C, similar to melting point due to rapid decay |
| Density | Estimated to be around 9.9 g/cm³ (predicted) |
| Electron Configuration | [Rn] 5f¹⁴ 7s² (predicted) |
| Oxidation States | +2, +3 (most stable is predicted to be +2) |
| Crystal Structure | Face-centered cubic (predicted) |
| Electronegativity | Pauling scale: 1.3 (estimated) |
| Ionization Energies | First: 641.6 kJ/mol (estimated) |
| Thermal Conductivity | Not determined |
| Magnetic Ordering | Not determined |
| Nuclear Property | Value for Nobelium |
|---|---|
| Atomic Number | 102 |
| Atomic Mass | ~259 u (most stable isotope Nobelium-259) |
| Radioactive | Yes, all isotopes have short half-lives |
| Oxidation States | +2, +3 (most stable for Nobelium) |
| Reactivity | Reactive, particularly in its +3 oxidation state |
| Ionization Energies | High |
| Compounds | Few known, Nobelium is mostly studied in its elemental form due to instability |
| Isotope | Half-life | Mode of Decay |
|---|---|---|
| Nobelium-253 | 1.62 minutes | Alpha decay |
| Nobelium-254 | 51 seconds | Alpha decay |
| Nobelium-255 | 3.1 minutes | Alpha decay |
| Nobelium-256 | 2.91 seconds | Alpha decay |
| Nobelium-257 | 25 seconds | Alpha decay |
| Nobelium-258 | 1.2 milliseconds | Alpha decay |
| Nobelium-259 | 58 minutes | Alpha decay |
| Nobelium-260 | 106 seconds | Alpha decay |
This article meticulously explored Nobelium, from its complex formation to its aqueous chemistry, highlighting its unique behavior in solutions and interactions with various ligands. Nobelium’s synthetic nature and placement in the actinide series underscore the challenges and insights into superheavy element research, offering a deeper understanding of the periodic table’s frontier and the intricate chemistry of transuranium elements.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical symbol for Nobelium?
No
Nb
Nm
Ne
What is the atomic number of Nobelium?
100
101
102
103
In which series of the periodic table is Nobelium found?
Lanthanide
Actinide
Transition metals
Halogens
Who was Nobelium named after?
Alfred Nobel
Marie Curie
Dmitri Mendeleev
Isaac Newton
What is the most stable isotope of Nobelium?
No-255
No-254
No-253
No-252
How was Nobelium first discovered?
By chemical reaction
By bombardment of curium with carbon ions
By neutron capture
By isolation from natural ores
In what year was Nobelium first discovered?
1950
1955
1958
19562
Which organization officially recognized the discovery of Nobelium?
International Union of Pure and Applied Chemistry (IUPAC)
American Chemical Society (ACS)
Royal Society of Chemistry (RSC)
National Institute of Standards and Technology (NIST)
Nobelium is most commonly produced in which type of facility?
Nuclear reactors
Particle accelerators
Chemical laboratories
Natural mines
What type of element is Nobelium classified as?
Alkali metal
Transition metal
Lanthanide
Actinide
Before you leave, take our quick quiz to enhance your learning!

