Which of the following is an example of an aromatic hydrocarbon?
Ethane
Benzene
Propane
Butane

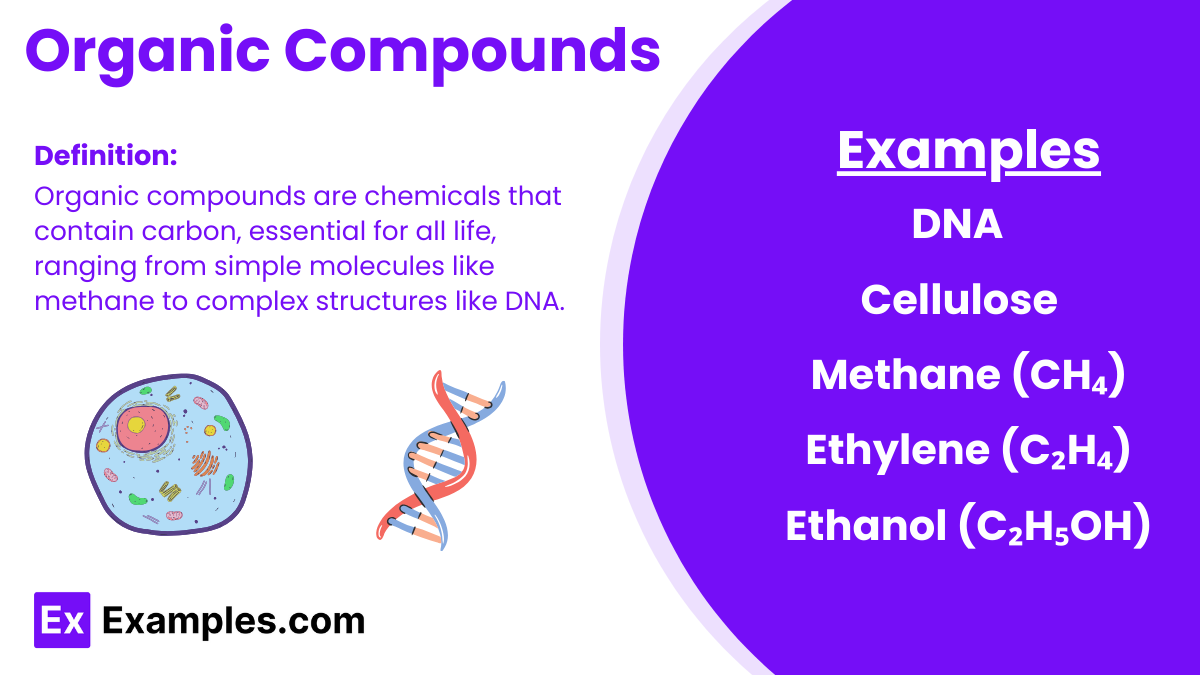
Organic compounds are a fascinating group of molecules that are primarily built around carbon atoms, bonded with elements like hydrogen, oxygen, nitrogen, and more. These compounds are the backbone of all living organisms, making up the structure of DNA, proteins, fats, and carbohydrates. In the world of chemistry, organic compounds are incredibly diverse, ranging from simple substances like methane to complex molecules like proteins and beyond. What makes them unique is their carbon-based structure, which allows for a vast array of chemical reactions, making them crucial not only for life processes but also in industries like pharmaceuticals, agriculture, and plastics
Organic compounds can be categorized into several main types based on their structures and the functional groups they contain. Understanding these types helps in studying their unique properties and uses:
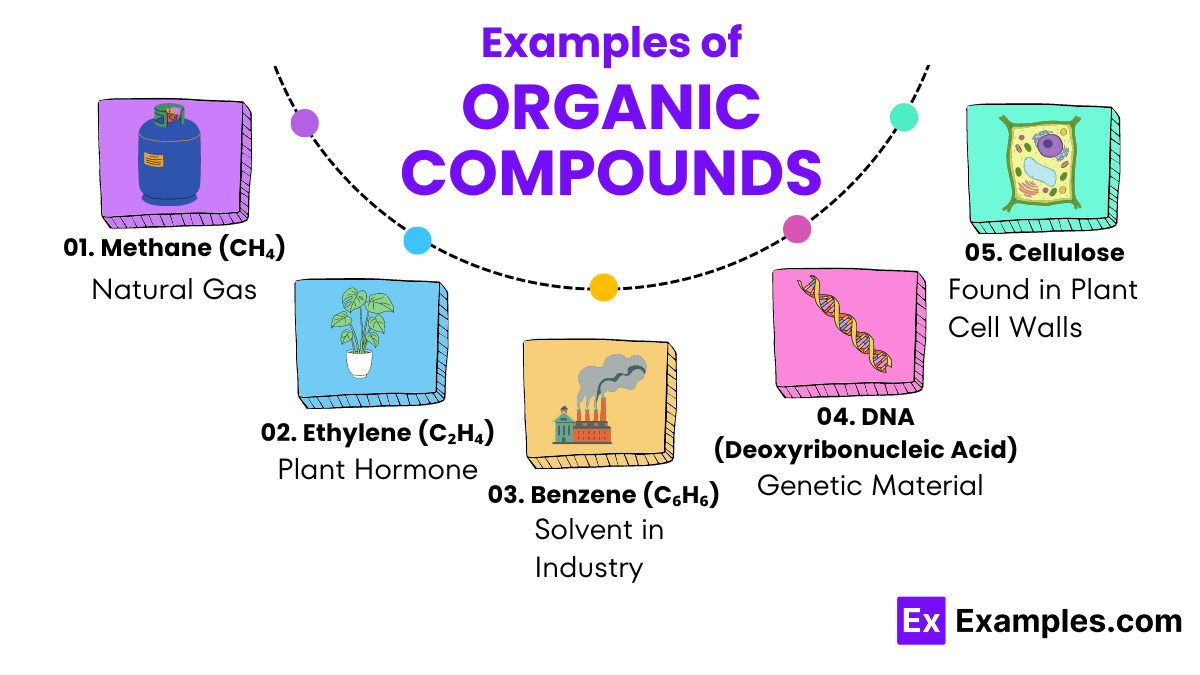
These are the simplest type of organic compounds, consisting entirely of carbon and hydrogen atoms with single bonds between them. Examples include methane and ethane. Alkanes are known for being major components of natural gas and petroleum.
Similar to alkanes, but alkenes contain at least one double bond between carbon atoms. This double bond changes the compound’s properties, making them more reactive. Common alkenes include ethylene, used extensively in the production of plastics.
These compounds contain at least one triple bond between carbon atoms, making them even more reactive than alkenes. An example is acetylene, which is used in welding torches for its high-temperature flame.
Aromatic compounds have a ring structure that includes alternating double and single bonds, known as aromatic rings. Benzene is a primary example, notable for its use in making various chemicals and drugs.
These contain one or more hydroxyl groups (-OH) attached to a carbon atom. Methanol and ethanol are well-known alcohols, with uses ranging from solvents to the alcohol found in beverages.
Featuring a carboxyl group (-COOH), these acids include substances like acetic acid (vinegar) and citric acid, which are key in food industries and biological processes.
The structure of organic compounds is centered around carbon atoms. Carbon is unique because it can form four stable bonds with other atoms, allowing it to create complex and varied structures. In organic compounds, carbon atoms are linked together in chains or rings, and these backbones are typically bonded with hydrogen, oxygen, nitrogen, or other elements. The simplest organic molecules, like methane (CH₄), consist of a single carbon atom bonded to four hydrogen atoms. As the structures become more complex, they can include double or triple bonds and may incorporate functional groups like alcohols, ketones, and acids, which significantly influence the properties and reactions of the compounds. This versatility makes organic chemistry a rich field of study, crucial for many industries and technologies.
Organic compounds are formed primarily through the bonding of carbon atoms with other elements like hydrogen, oxygen, nitrogen, and sulfur. This process begins with carbon’s ability to form stable, covalent bonds due to its four valence electrons, which allows it to bond with up to four other atoms. This unique characteristic leads to a vast array of molecular structures and complexities.
In nature, organic compounds are produced by living organisms through various biochemical processes. For example, plants convert carbon dioxide from the air into glucose through photosynthesis, a fundamental organic process. In industrial settings, organic compounds can be synthesized in laboratories and factories, using techniques such as chemical reactions involving catalysts, heat, and pressure to bond carbon with other elements. This synthetic approach allows for the creation of numerous useful organic compounds not readily available in nature, such as plastics, pharmaceuticals, and dyes.
| Property | Description |
|---|---|
| Boiling Point | Varies from low to high |
| Melting Point | Ranges from very low to high |
| Solubility | Soluble in organic solvents, less in water |
| Odor | Many have distinctive smells |
| State | Can be gases, liquids, or solids |
| Volatility | Varies; many are volatile |
| Flammability | Often highly flammable |
The boiling point of organic compounds can vary significantly. Simple molecules like methane have very low boiling points, while larger, more complex molecules such as fatty acids have high boiling points.
Melting points in organic compounds also show a wide range. For instance, methane is a gas at room temperature, whereas substances like wax are solid, with a relatively high melting point.
Organic compounds tend to be soluble in organic solvents (like acetone or ethanol) due to similar intermolecular forces but are less soluble in water. This principle is often summarized by the phrase “like dissolves like.”
Many organic compounds have distinctive odors. For example, benzene has a sweet odor, and ethanoic acid smells like vinegar because it is the main component of vinegar.
The state of an organic compound at room temperature can be gas, liquid, or solid. Methane is a gas, ethanol is a liquid, and naphthalene appears as a solid.
Volatility refers to the ability of a substance to vaporize. Ethanol, for instance, is more volatile than water, which means it evaporates more quickly.
Organic compounds, particularly those with a high proportion of carbon and hydrogen, tend to be highly flammable. This makes them useful as fuels but also requires careful handling.
Organic compounds predominantly consist of covalent bonds, where electrons are shared between atoms. This type of bonding generally results in stronger and more stable molecular structures.
The backbone of all organic compounds is carbon. Carbon’s unique ability to form four stable bonds with other atoms, including other carbon atoms, allows for the formation of complex and diverse molecular structures.
Organic molecules often contain functional groups—specific groups of atoms that behave in predictable ways. Examples include hydroxyl groups (-OH) in alcohols and carboxyl groups (-COOH) in acids. These groups largely determine the chemical properties and reactions of the compounds.
Organic compounds are typically more soluble in organic solvents (like benzene, ether, or alcohols) than in water. This characteristic is due to the non-polar nature of many organic compounds, which does not mix well with water, a polar solvent.
Many organic compounds are volatile, meaning they can evaporate at relatively low temperatures. This is particularly true for those with low molecular weights, such as methane and ethylene.
Organic compounds can generally burn or combust, producing carbon dioxide, water, and other gases. This combustibility makes organic compounds excellent fuels.
Organic compounds can exhibit isomerism, where compounds with the same molecular formula have different structural arrangements. This results in different physical and chemical properties, which is crucial for the diversity of organic chemistry.
The molecular complexity of organic compounds allows for a vast array of structures, from simple gases like methane to complex polymers and biological molecules like DNA and proteins.
While it’s challenging to pinpoint a single “most important” organic compound due to the vast diversity and essential roles of these molecules in various biological and industrial processes, water (H₂O) often stands out. Although not typically classified as an organic compound because it lacks carbon, water is crucial for the existence of life and the biochemical processes involving organic compounds. In the realm of strictly organic chemistry, compounds like glucose and DNA (Deoxyribonucleic Acid) are fundamental. Glucose serves as a primary energy source for living organisms, while DNA carries genetic information critical for life functions.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which of the following is an example of an aromatic hydrocarbon?
Ethane
Benzene
Propane
Butane
What functional group is present in alcohols?
Hydroxyl group (-OH)
Carbonyl group (C=O)
Carboxyl group (-COOH)
Amino group (-NH2)
Which type of reaction involves the breaking of a molecule into smaller units by the addition of water?
Dehydration
Hydrolysis
Hydrogenation
Oxidation
What is the main characteristic of a ketone?
Presence of a carboxyl group
Presence of a hydroxyl group
Presence of a carbonyl group bonded to two carbon atoms
Presence of an amino group
What is the product of the complete combustion of an alkane?
Carbon monoxide and water
Carbon dioxide and water
Carbon dioxide and hydrogen
Carbon monoxide and hydrogen
What type of isomerism is exhibited by 1-butene and 2-butene?
Structural isomerism
Geometric isomerism
Optical isomerism
Functional isomerism
What is the functional group present in carboxylic acids?
Hydroxyl group
Carbonyl group
Carboxyl group
Amino group
What type of reaction is esterification?
Addition reaction
Substitution reaction
Elimination reaction
Condensation reaction
What is the name of the reaction in which an alkene reacts with hydrogen to form an alkane?
Halogenation
Hydrogenation
Hydrolysis
Hydration
Which organic compound is commonly used as a solvent in nail polish remover?
Methanol
Ethanol
Acetone
Benzene
Before you leave, take our quick quiz to enhance your learning!

