What is the atomic number of Phosphorus?
12
15
16
14

Explore the vibrant world of Phosphorus, an element that ignites curiosity and innovation in the scientific community. This guide is your pathway to understanding its intricate nature, practical uses, and why it’s often paired with Hydrogen in various reactions and applications. Dive into examples that illuminate its role in nature and technology, making your teachings about Phosphorus as radiant as the element itself. Perfect for educators looking to enrich their knowledge and spark student interest!
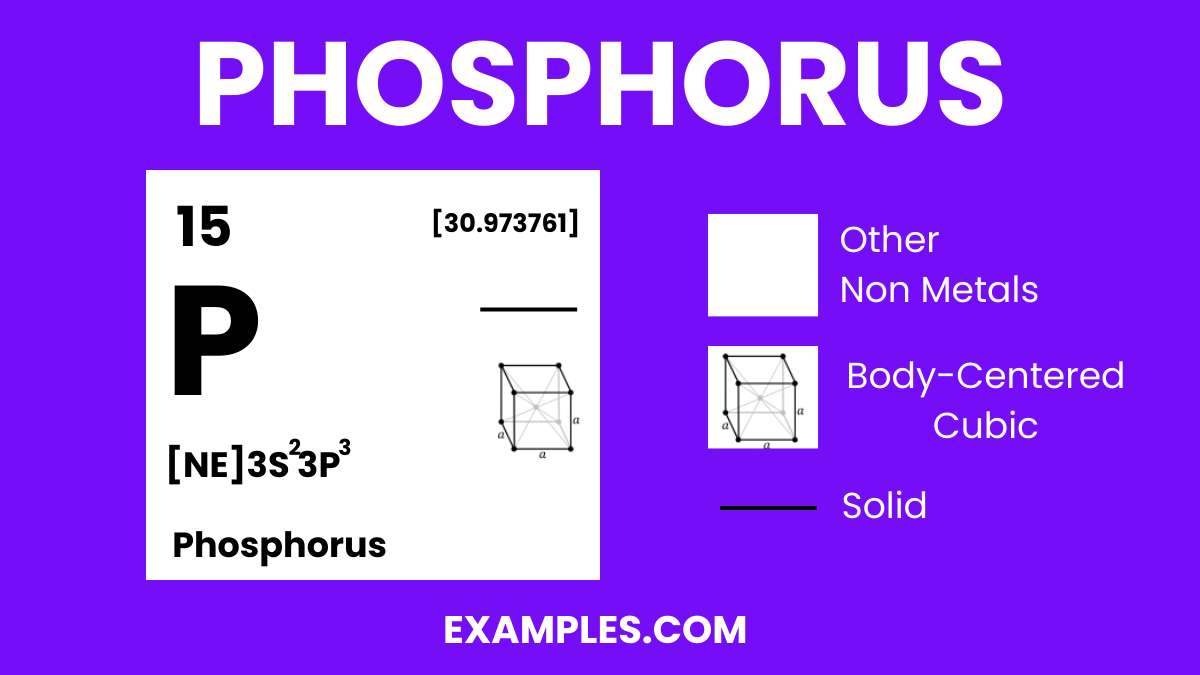
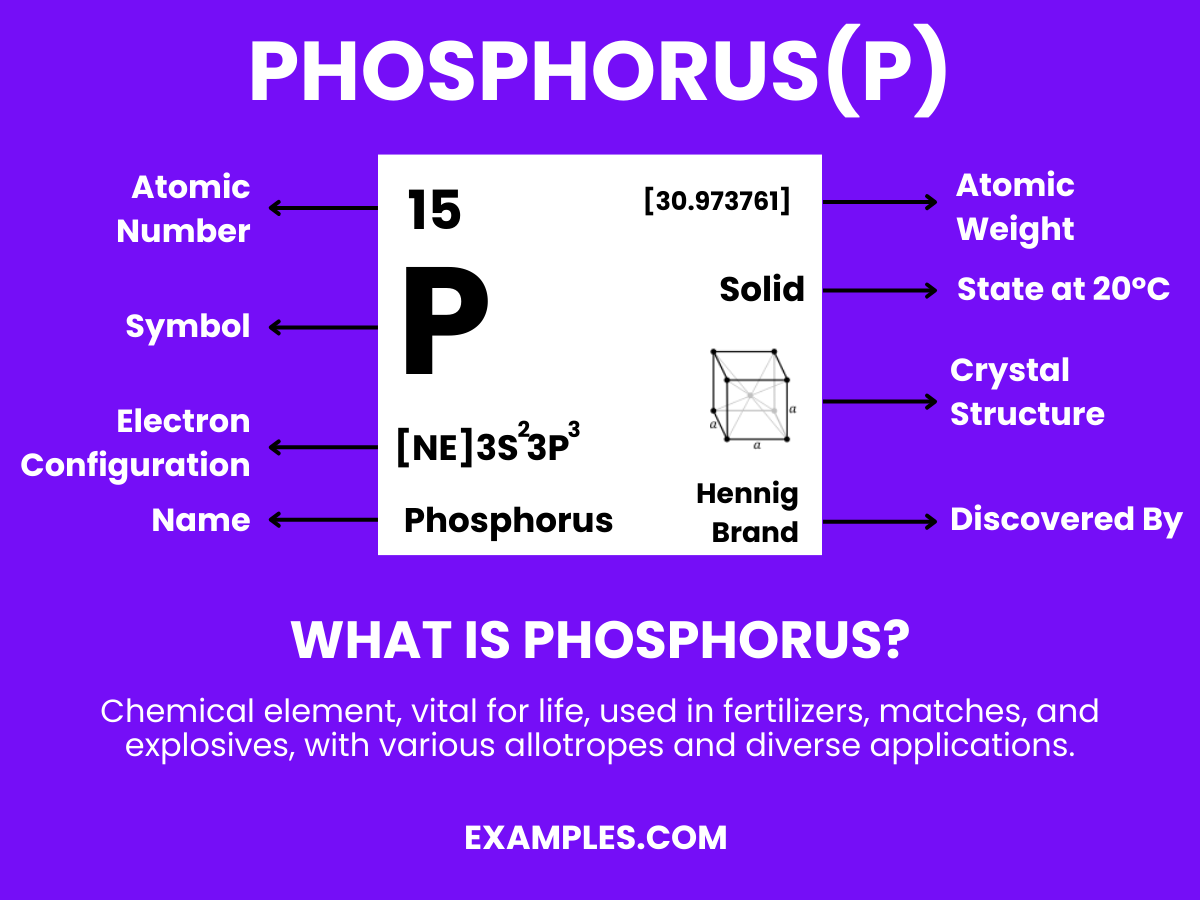
Phosphorus is a chemical element with the symbol P and atomic number 15. It is a non-metal that typically exists in two major forms—white and red phosphorus—each with distinct properties and uses. As an essential element in life, it plays a critical role in biological processes, including the formation of DNA, cell membranes, and bone structure. It’s also widely used in industry for creating fertilizers, safety matches, and other products. Understanding Phosphorus is key to comprehending various chemical reactions and natural phenomena.
| Hydrogen | Sulfur |
| Carbon | Chlorine |
| Nitrogen | Selenium |
| Oxygen | Bromine |
| Fluorine | Iodine |
Phosphorus is known for its high reactivity, especially white phosphorus. It exhibits various chemical behaviors:
These properties make phosphorus a highly valuable and versatile element in various chemical processes, including fertilizers, safety matches, pesticides, and more. Its ability to react and form a wide range of compounds is fundamental to its extensive use in industrial and household applications.
| Property | Value with Unit |
|---|---|
| Boiling Point (white P) | 280.5 °C |
| Melting Point (white P) | 44.15 °C |
| Heat of Vaporization | 51.9 kJ/mol (white P) |
| Heat of Fusion | 2.51 kJ/mol (white P) |
| Specific Heat Capacity (white P, 25°C) | 0.769 J/g·K |
| Thermal Conductivity (white P) | 0.236 W/m·K |
| Property | Value with Unit |
|---|---|
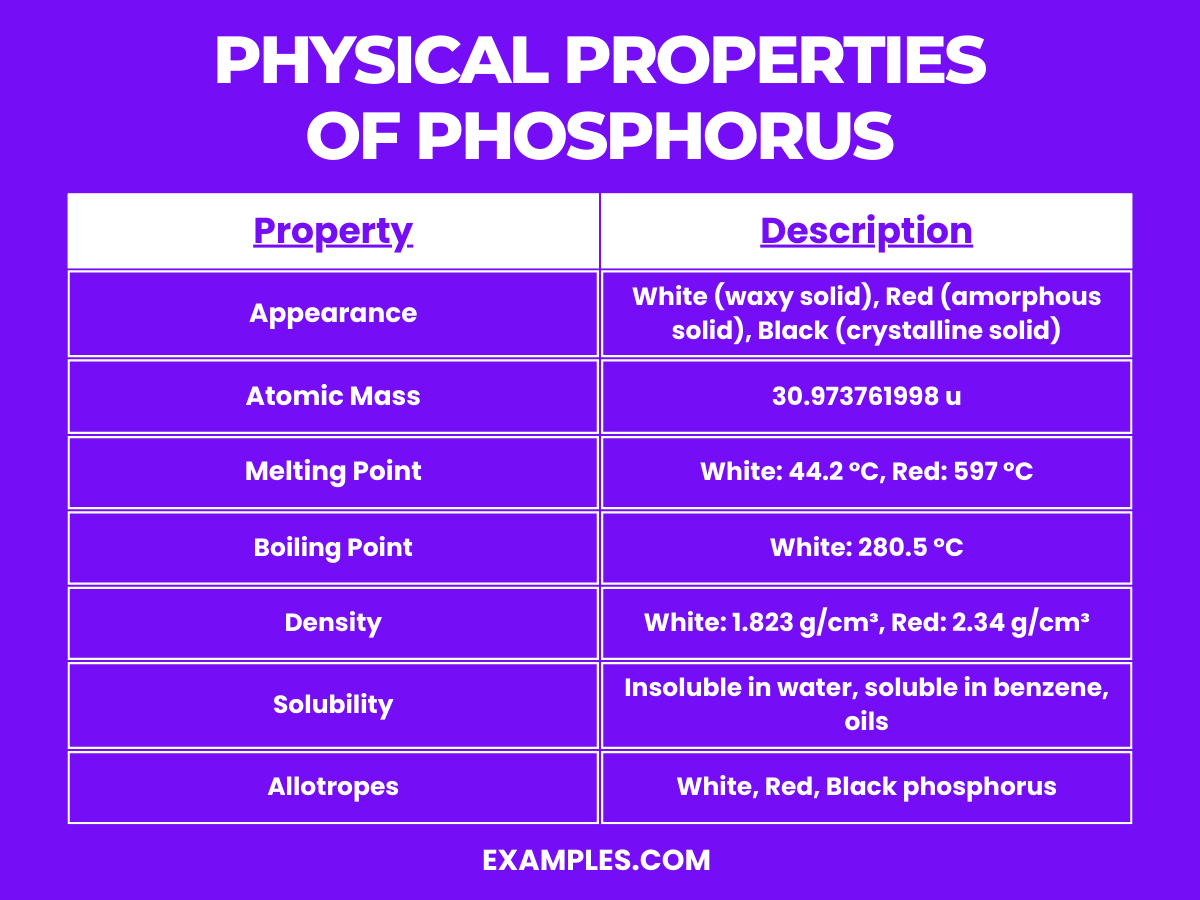
| Density (white P, 20°C) | 1.823 kg/m³ |
| Solubility in Water | Insoluble (white P) |
| Mohs Hardness (white P) | 0.5 |
| Crystal Structure (white P) | Cubic |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (white P) | High (Insulator) |
| Electronegativity (Pauling scale) | 2.19 |
| Electron Affinity | 72 kJ/mol |
| Property | Value with Unit |
|---|---|
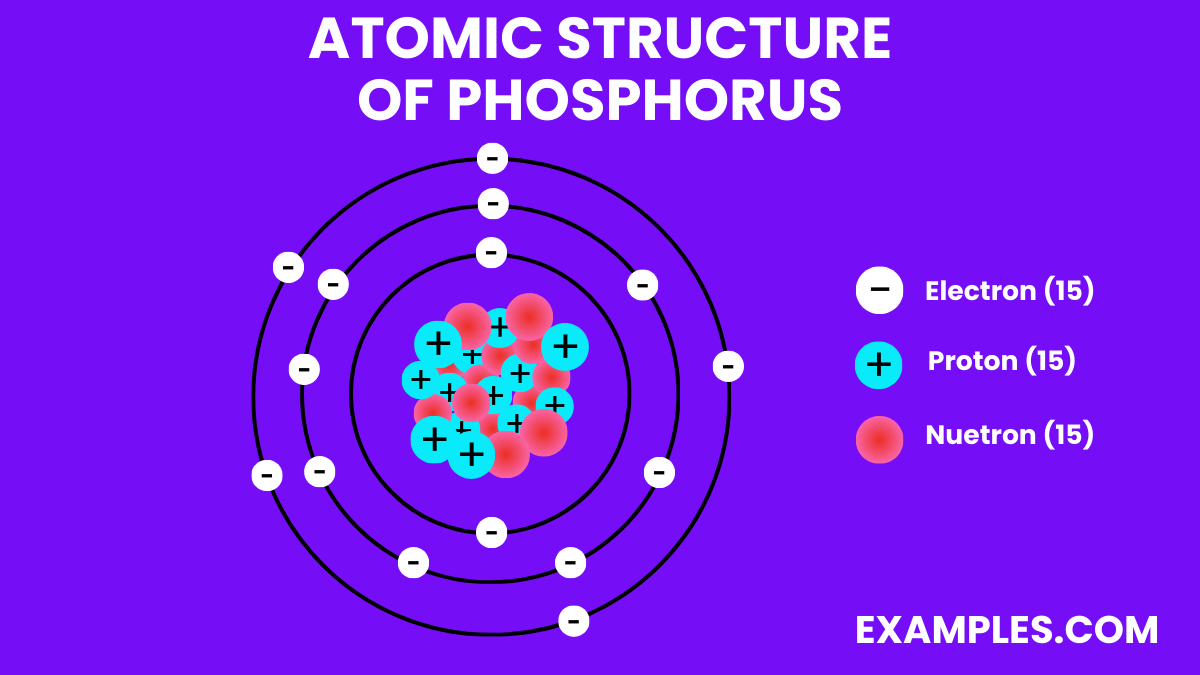
| Atomic Number | 15 |
| Atomic Mass | 30.973762 amu |
| Isotopes | ^31P (100%) |
| Nuclear Spin (for ^31P) | 1/2 ℏ |
| Neutron Cross Section (for ^31P) | 0.172 barns |
| Nuclear Magnetic Moment (for ^31P) | 1.1316 µN |
Phosphorus is unique due to its several allotropes, mainly white, red, and black phosphorus, each with distinct physical and chemical properties. Understanding these allotropes is crucial as they determine the element’s reactivity and use in various applications.
Appearance and Properties: White phosphorus consists of P₄ tetrahedra, where each atom is bonded to the other three. It’s waxy, white, and highly reactive, especially with oxygen. It must be stored underwater to prevent it from igniting in air.
Reactivity: White phosphorus is highly reactive and is used in incendiary devices and smoke screens. It can spontaneously ignite in air at around 30°C.
Equation Example: When white phosphorus reacts with chlorine, phosphorus trichloride is formed. P₄+6Cl₂→4PCl₃
Appearance and Properties: Red phosphorus is more stable and safer to handle than white phosphorus. It’s an amorphous network that lacks the P₄ structure.
Uses: It’s used in safety matches, fireworks, and other pyrotechnics. Unlike white phosphorus, it doesn’t ignite in air and is less toxic.
Conversion from White Phosphorus: White phosphorus can be converted to red phosphorus by heating under controlled conditions.
Appearance and Properties: Black phosphorus is the least reactive allotrope and has a layered structure, similar to graphite. It’s a good conductor of electricity.
Uses: Due to its unique electrical and mechanical properties, it’s researched for use in electronics, semiconductors, and batteries.
Formation: It’s obtained by heating white phosphorus under high pressures.
Understanding these allotropes allows for better utilization and handling of phosphorus in various industrial and scientific applications. Each allotrope’s unique properties cater to specific needs, making phosphorus a versatile and widely used element in the modern world. Whether it’s in the manufacturing of safety matches or the potential future of electronics with black phosphorus, this element’s varied forms are integral to numerous sectors.
Phosphorus forms a wide array of compounds, each with its unique properties and applications. The versatility of phosphorus in forming compounds lies in its ability to bond with many different elements, including oxygen, hydrogen, and various metals. Here’s an in-depth look at some of the most significant phosphorus compounds:
Phosphates are perhaps the most well-known compounds of phosphorus, essential in biochemistry, agriculture, and industry. They are formed by the combination of phosphoric acid with metals. One of the most common phosphates is calcium phosphate, used in fertilizers and as a nutritional supplement.
Equation: 3Ca(OH)₂+2H₃PO₄→Ca₃(PO₄)₂+6H₂O (Formation of Calcium Phosphate)
Phosphoric acid is a weak acid that forms when phosphorus pentoxide reacts with water. It’s widely used in soft drinks, as a rust remover, and in fertilizers.
Equation: P4O₁₀+6H₂O→4H₃PO₄ (Formation of Phosphoric Acid)
Phosphorus forms a series of halides, the most common being phosphorus trichloride (PCl₃) and phosphorus pentachloride (PCl₅). These compounds are primarily used in organic synthesis and as chlorinating agents.
Equations: 2P+3Cl₂→2PCl₃ (Formation of Phosphorus Trichloride) P+5Cl₂→PCl₅ (Formation of Phosphorus Pentachloride)
Phosphine is a toxic and flammable gas formed when calcium phosphide reacts with water or acid. It’s used as a fumigant and in the semiconductor industry.
Equation: Ca₃P₂+6H₂O→2PH₃+3Ca(OH)₂ (Formation of Phosphine)
Organophosphates are widely used as insecticides and nerve agents. These compounds work by disrupting the nervous system’s function and are derived from phosphoric acid. A common example is glyphosate, a widely used herbicide.
Equation: C₃H₈NO₅P+H₂O→(Glyphosatehydrolysis)
By understanding these compounds and their reactions, one can appreciate the vital role of phosphorus in various fields, from agriculture and biochemistry to industrial applications. The unique chemical behavior of phosphorus allows it to form a wide array of compounds, each serving important functions in the world around us. As we continue to study and harness these compounds, phosphorus remains at the forefront of scientific and industrial innovation.
Phosphorus has several isotopes, with Phosphorus-31 being the most stable and abundant in nature. Here’s a table describing some of the isotopes of Phosphorus:
| Isotope | Atomic Mass | Half-Life | Decay Mode | Natural Abundance |
|---|---|---|---|---|
| Phosphorus-31 | 30.97376199 | Stable | — | 100% |
| Phosphorus-32 | 31.97390727 | 14.28 days | Beta decay | Trace |
| Phosphorus-33 | 32.9717255 | 25.3 days | Beta decay | Trace |
Phosphorus-31 is the only stable isotope and has significant applications in nuclear magnetic resonance (NMR) due to its nuclear spin properties. Phosphorus-32 and Phosphorus-33 are radioactive and used in various scientific and medical applications, including tracers and treatment of specific types of cancer.
Phosphorus is a versatile element with numerous applications across various fields. Here are some of the most significant uses of phosphorus:
Phosphorus plays a pivotal role in the biological and physiological functions of all living organisms. Here are the key points detailing its significance:
By understanding these roles, it’s evident that phosphorus is not merely an element on the periodic table but a cornerstone of life and vitality.
The commercial production of phosphorus primarily involves the extraction and processing of phosphate rock. The process is optimized for efficiency, environmental impact, and quality of the final product. Here’s how phosphorus is commercially produced:
In commercial settings, phosphorus is primarily used to produce phosphoric acid, a precursor to fertilizers, and various other phosphorus compounds that serve as flame retardants, pesticides, and in numerous other applications. The production is continuously optimized for higher yields, better quality, and reduced environmental impact, reflecting the critical nature of this element in a wide array of industrial and agricultural processes.
Phosphorus is a vital element in the human body and plays several key roles in maintaining health and well-being. However, both deficiency and excess of phosphorus can lead to health issues:
Phosphorus, while essential for life, can have profound environmental impacts, especially when its natural balance is disrupted:
The chemical name for phosphorus is simply “Phosphorus” with the chemical symbol P and atomic number 15.
Phosphorus is used in fertilizers, detergents, pesticides, and is vital for DNA, bones, and energy transfer in living organisms.
Phosphorus exists mainly as P4 in its elemental form, especially as white phosphorus, which consists of P4 tetrahedra.
Phosphorus is primarily obtained from phosphate rocks and is widely distributed in the earth’s crust and living organisms.
Phosphorus is critical for life, involved in DNA, energy transfer, bone health, and essential in agriculture and industry.
Phosphorus is an essential element, intricately woven into the tapestry of life and industry. Understanding its properties, uses, and impact is crucial for students, educators, and professionals. By mastering how to write and convey its importance, one can appreciate Phosphorus’s role in the natural and scientific world, leading to more informed, sustainable decisions and innovative applications.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Phosphorus?
12
15
16
14
Which allotrope of Phosphorus is commonly used in safety matches?
White phosphorus
Red phosphorus
Black phosphorus
Purple phosphorus
Phosphorus is essential for life primarily due to its role in:
Bone structure
DNA and RNA structure
Cell membrane structure
All of the above
The primary source of industrial phosphorus is:
Bone ash
Phosphate rock
Plant extracts
Animal wastes
Excessive use of phosphorus-rich fertilizers can lead to:
Increased crop yield
Eutrophication
Reduced pesticide need
Improved soil health
Which property of white phosphorus is the reason for its severe toxicity?
It is highly reactive
It is radioactive
It is stable
It is inert
Which process is used to convert phosphate rock into soluble phosphate, usable by plants?
Hydrolysis
Nitrification
The Haber process
The wet acid process
Phosphorus was first isolated by:
Antoine Lavoisier
Henry Moseley
Hennig Brand
Marie Curie
Phosphorus helps plants convert other nutrients into usable building blocks through:
Photosynthesis
Respiration
ATP production
Transpiration
The change of phosphorus from phosphate rock to soluble form primarily affects which environmental compartment?
Air
Soil
Water
Flora
Before you leave, take our quick quiz to enhance your learning!

