What is the chemical formula of Potassium Chloride?
KCl
KClO3
KClO4
KClO2

Potassium Chloride (KCl) is an ionic compound and a metal halide salt composed of potassium and chlorine, integral to the field of chemistry. Potassium chloride finds extensive use in diverse industries and fields such as medicine, food processing, and agriculture due to its wide range of applications. In its pure form, KCl appears as odorless, colorless crystals or granules with a salty taste. This compound is essential for its role in human, animal, and plant physiology, serving as a vital source of potassium in dietary supplements and fertilizers. Potassium is a key electrolyte in the body, crucial for maintaining fluid balance, nerve signals, and muscle function. In agriculture, KCl serves as a potassium-based fertilizer, boosting crop yield and improving quality. Additionally, it finds applications in the food industry as a salt substitute, and in science and manufacturing as a source of potassium ions for various chemical processes in chemistry.
Potassium Chloride (KCl) is a simple, yet vital chemical compound that plays a significant role in various aspects of daily life. It is an ionic compound composed of potassium (K) and chlorine (Cl) atoms. It exists as colorless, odorless crystals with a salty taste. While naturally occurring in minerals and saltwater, KCl is also manufactured for various purposes. In our bodies, Potassium Chloride is crucial for keeping our muscles working correctly, ensuring our nerves send signals the way they should, and helping our cells maintain the right balance of fluids. Apart from its biological importance, KCl plays a vital role in agriculture as a crucial component of fertilizers, supplying plants with essential potassium nutrients to ensure strong growth. Additionally, KCl serves as a substitute for salt in food processing, enabling a reduction in sodium intake without sacrificing flavor. In the industrial and scientific world, Potassium Chloride is a key ingredient in manufacturing processes and laboratory procedures, used for its chemical properties, including its ability to conduct electricity when dissolved in water.
| Formula | KCl |
|---|---|
| Hill Formula | ClK |
| Name | Potassium Chloride |
| Alternate Names | Enseal, Kalitabs, Kaochlor, Pfiklor, Potavescent, Rekawan |
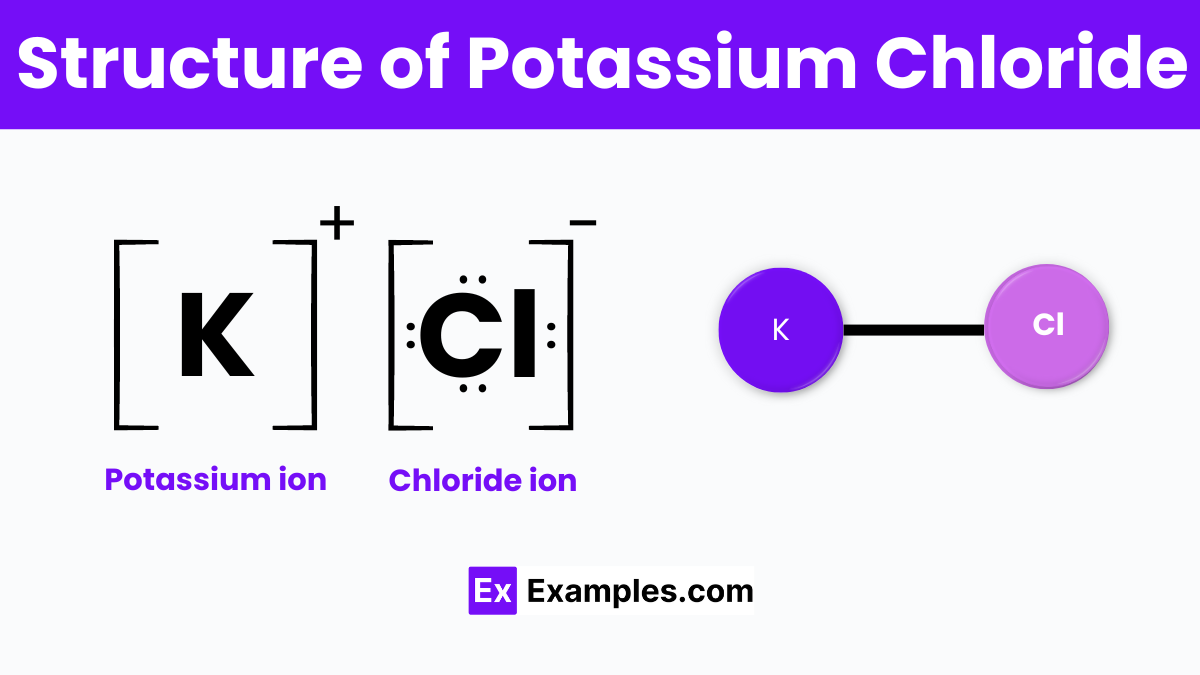
The structure of Potassium Chloride (KCl) is a classic example of an ionic compound‘s crystalline lattice arrangement. This chemical compound is formed when potassium (K) atoms donate their outermost electron to chlorine (Cl) atoms, resulting in the formation of potassium ions (K⁺) and chloride ions (Cl⁻). These ions then attract each other due to their opposite charges, creating a strong ionic bond. In its solid state, KCl adopts a cubic lattice structure known as the face-centered cubic (FCC) crystal system. In this structure, each potassium ion is surrounded by six chloride ions, and similarly, each chloride ion is surrounded by six potassium ions.. This symmetrical arrangement allows for a highly stable and tightly packed crystal lattice.
The cubic lattice structure of Potassium Chloride contributes to its high melting point and boiling point, as well as its ability to dissolve well in water. When KCl dissolves, the ionic bonds between the potassium and chloride ions break, and the ions disperse throughout the water, making it an excellent conductor of electricity.
Potassium Chloride (KCl) is produced using various methods, both natural and synthetic. In nature, KCl can be obtained through mining, where it’s extracted from underground deposits, or through the evaporation of water from locations rich in KCl, such as the Dead Sea. These natural methods provide sources for KCl used in various industries.
Additionally, in laboratory settings, KCl can be synthesized through a straightforward reaction. This involves mixing potassium hydroxide (KOH) with hydrochloric acid (HCl), resulting in the formation of potassium chloride (KCl) along with water (H₂O). This synthetic production method is often employed for scientific research and specific industrial applications where high-purity KCl is required. The balanced Equation is:
After obtaining KCl, it often requires cleaning to remove any impurities mixed with it. This process typically entails dissolving the KCl in hot water, allowing it to cool, and then separating the pure KCl crystals from the water. The choice of purification method depends on factors like the availability of natural resources, the desired purity level, and economic considerations. Each method has its advantages and limitations, influencing its application in various industries.
| Property | Description |
|---|---|
| Appearance | Colorless or white crystals |
| Odor | Odorless |
| Taste | Salty |
| State at Room Temperature | Solid |
| Melting Point | 770°C (1,418°F) |
| Boiling Point | 1,420°C (2,588°F) |
| Solubility in Water | Soluble (34.4 g/100 mL at 20°C) |
| Density | 1.98 g/cm³ (solid) |
| Crystal Structure | Face-centered cubic (FCC) |
| Molecular Weight | 74.55 g/mol |
KCl dissolves in water, separating into potassium (K⁺) and chloride (Cl⁻) ions, making the solution conduct electricity.
Equation: KCl (solid) → K⁺ (in water) + Cl⁻ (in water).
When mixed with silver nitrate (AgNO₃), KCl forms white silver chloride (AgCl) and potassium nitrate (KNO₃)
Equation: KCl + AgNO₃ → AgCl (solid) + KNO₃.
Electrolysis of a KCl solution produces potassium hydroxide (KOH), chlorine gas (Cl₂), and hydrogen gas (H₂)
Equation: 2KCl + 2H₂O (electrolysis) → 2KOH + Cl₂ + H₂.
KCl reacts with sulfuric acid (H₂SO₄) to create hydrochloric acid (HCl) and potassium sulfate (K₂SO₄)
Equation: 2KCl + H₂SO₄ → 2HCl + K₂SO₄.
| Property | Identifier |
|---|---|
| CAS Registry Number | 7447-40-7 |
| PubChem Compound ID | 4873 |
| PubChem Substance ID | 24852090 |
| SMILES Identifier | [Cl-].[K+] |
| InChI Identifier | InChI=1/ClH.K/h1H;/q;+1/p-1/fCl.K/h1h;/q-1;m |
| InChI Key | WCUXLLCKKVVCTQ-UHVCZDMOCF |
| RTECS Number | TS8050000 |
| MDL Number | MFCD00011360 |
| Property | Rating |
|---|---|
| NFPA Health Rating | 1 |
| NFPA Fire Rating | 0 |
| NFPA Reactivity Rating | 0 |
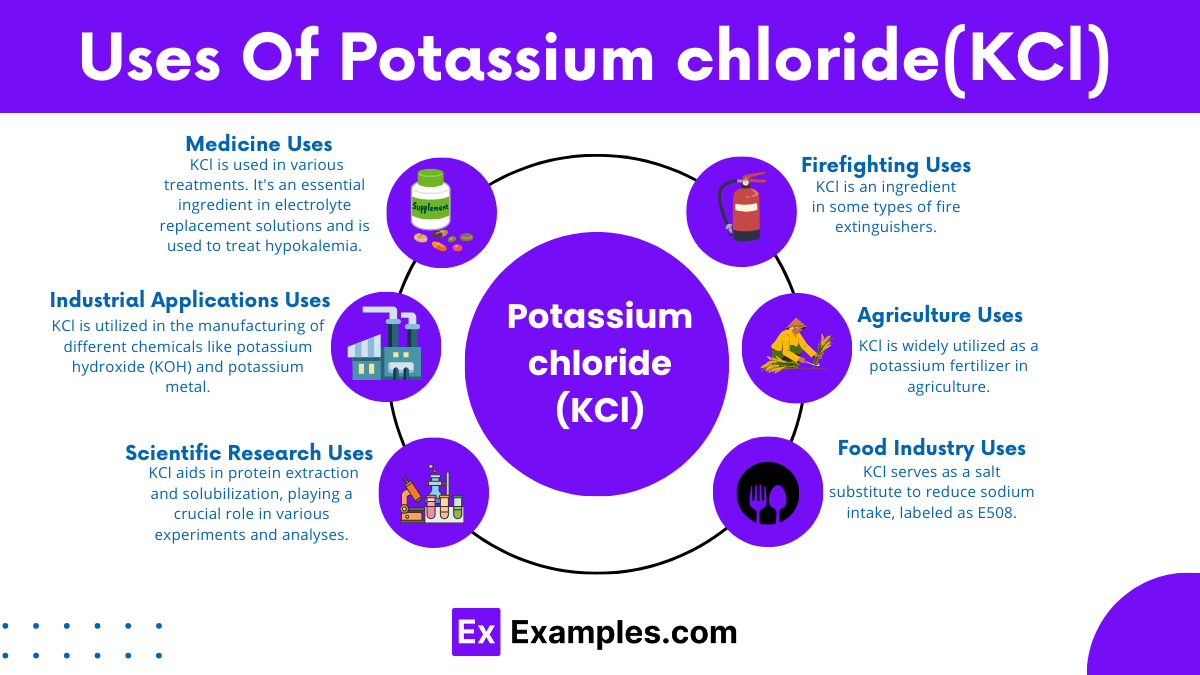
KCl is widely utilized as a potassium fertilizer in agriculture. Potassium is a crucial nutrient for plant growth, helping in water regulation, the synthesis of proteins, and the activation of enzymes.
In the medical field, KCl is used in various treatments. It’s an essential ingredient in electrolyte replacement solutions and is used to treat hypokalemia, a condition caused by low levels of potassium in the blood. In some cases, it’s used in lethal injections.
As a food additive, KCl serves as a salt substitute to reduce sodium intake, labeled as E508. Potassium chloride (KCl) is utilized in low-sodium and “salt-free” products, assisting individuals in managing blood pressure and promoting heart health.
KCl is utilized in the manufacturing of different chemicals like potassium hydroxide (KOH) and potassium metal. Moreover, it is employed in metal electroplating due to its conductivity.
KCl solutions are employed in laboratories as calibration standards and are extensively utilized in scientific research, especially within the realm of molecular biology. In this field, KCl aids in protein extraction and solubilization, playing a crucial role in various experiments and analyses.
KCl is an ingredient in some types of fire extinguishers, specifically in class potassium extinguishers used for cooking oil or fat fires.
KCl is a significant source of potassium, an essential nutrient that supports heart health, muscle function, and nerve conduction. Using KCl as a supplement can help maintain optimal potassium levels in the body.
As a salt substitute, KCl allows individuals to lower their sodium intake without sacrificing taste. This can be particularly beneficial for people with high blood pressure, heart disease, or kidney problems.
Potassium chloride (KCl) serves as a fertilizer, supplying potassium to enhance crop yield and quality. Potassium helps plants resist drought, disease, and pests, and supports overall plant health.
Potassium chloride (KCl) plays a vital role in treating and preventing potassium deficiency, medically termed hypokalemia. Moreover, it is included in specific medications and treatments, offering crucial support in healthcare settings
Potassium chloride (KCl) finds utility in the manufacturing of various chemicals, including potassium hydroxide and potassium metal. Its properties also make it useful in metal electroplating and as a flux in welding.
Potassium chloride (KCl) solutions serve as essential calibration standards for laboratory instruments in scientific investigations. It’s also crucial in molecular biology for protein studies and DNA extraction processes.
Consuming KCl, especially in supplement form, can lead to gastrointestinal discomfort. This includes symptoms like nausea, vomiting, diarrhea, and abdominal pain.
Some people may experience an unpleasant taste in their mouth after consuming KCl, particularly when used as a salt substitute.
While potassium chloride (KCl) serves as a remedy for potassium deficiency, its improper administration may result in hyperkalemia, an ailment marked by an excess of potassium in the bloodstream. Symptoms of hyperkalemia include muscle weakness, fatigue, irregular heart rhythms, and in severe cases, cardiac arrest.
Individuals with kidney problems need to be cautious with their potassium intake, including KCl, as their kidneys may not be able to remove excess potassium efficiently, leading to an increased risk of hyperkalemia.
While uncommon, certain individuals may experience an allergic reaction to KCl, which can result in symptoms such as itching, rash, or difficulty breathing.
Potassium chloride is beneficial for maintaining electrolyte balance and nerve function, but moderation is key due to potential side effects.
Take potassium chloride as prescribed by a healthcare provider, usually for electrolyte imbalances or low potassium levels.
Low potassium can cause muscle aches, cramps, and weakness, significantly impacting overall physical well-being.

Potassium Chloride (KCl) is an ionic compound and a metal halide salt composed of potassium and chlorine, integral to the field of chemistry. Potassium chloride finds extensive use in diverse industries and fields such as medicine, food processing, and agriculture due to its wide range of applications. In its pure form, KCl appears as odorless, colorless crystals or granules with a salty taste. This compound is essential for its role in human, animal, and plant physiology, serving as a vital source of potassium in dietary supplements and fertilizers. Potassium is a key electrolyte in the body, crucial for maintaining fluid balance, nerve signals, and muscle function. In agriculture, KCl serves as a potassium-based fertilizer, boosting crop yield and improving quality. Additionally, it finds applications in the food industry as a salt substitute, and in science and manufacturing as a source of potassium ions for various chemical processes in chemistry.
Potassium Chloride (KCl) is a simple, yet vital chemical compound that plays a significant role in various aspects of daily life. It is an ionic compound composed of potassium (K) and chlorine (Cl) atoms. It exists as colorless, odorless crystals with a salty taste. While naturally occurring in minerals and saltwater, KCl is also manufactured for various purposes. In our bodies, Potassium Chloride is crucial for keeping our muscles working correctly, ensuring our nerves send signals the way they should, and helping our cells maintain the right balance of fluids. Apart from its biological importance, KCl plays a vital role in agriculture as a crucial component of fertilizers, supplying plants with essential potassium nutrients to ensure strong growth. Additionally, KCl serves as a substitute for salt in food processing, enabling a reduction in sodium intake without sacrificing flavor. In the industrial and scientific world, Potassium Chloride is a key ingredient in manufacturing processes and laboratory procedures, used for its chemical properties, including its ability to conduct electricity when dissolved in water.
Formula | KCl |
|---|---|
Hill Formula | ClK |
Name | Potassium Chloride |
Alternate Names | Enseal, Kalitabs, Kaochlor, Pfiklor, Potavescent, Rekawan |

The structure of Potassium Chloride (KCl) is a classic example of an ionic compound‘s crystalline lattice arrangement. This chemical compound is formed when potassium (K) atoms donate their outermost electron to chlorine (Cl) atoms, resulting in the formation of potassium ions (K⁺) and chloride ions (Cl⁻). These ions then attract each other due to their opposite charges, creating a strong ionic bond. In its solid state, KCl adopts a cubic lattice structure known as the face-centered cubic (FCC) crystal system. In this structure, each potassium ion is surrounded by six chloride ions, and similarly, each chloride ion is surrounded by six potassium ions.. This symmetrical arrangement allows for a highly stable and tightly packed crystal lattice.
The cubic lattice structure of Potassium Chloride contributes to its high melting point and boiling point, as well as its ability to dissolve well in water. When KCl dissolves, the ionic bonds between the potassium and chloride ions break, and the ions disperse throughout the water, making it an excellent conductor of electricity.
Potassium Chloride (KCl) is produced using various methods, both natural and synthetic. In nature, KCl can be obtained through mining, where it’s extracted from underground deposits, or through the evaporation of water from locations rich in KCl, such as the Dead Sea. These natural methods provide sources for KCl used in various industries.
Additionally, in laboratory settings, KCl can be synthesized through a straightforward reaction. This involves mixing potassium hydroxide (KOH) with hydrochloric acid (HCl), resulting in the formation of potassium chloride (KCl) along with water (H₂O). This synthetic production method is often employed for scientific research and specific industrial applications where high-purity KCl is required. The balanced Equation is:
KOH + HCl → KCl + H₂O
After obtaining KCl, it often requires cleaning to remove any impurities mixed with it. This process typically entails dissolving the KCl in hot water, allowing it to cool, and then separating the pure KCl crystals from the water. The choice of purification method depends on factors like the availability of natural resources, the desired purity level, and economic considerations. Each method has its advantages and limitations, influencing its application in various industries.
Property | Description |
|---|---|
Appearance | Colorless or white crystals |
Odor | Odorless |
Taste | Salty |
State at Room Temperature | Solid |
Melting Point | 770°C (1,418°F) |
Boiling Point | 1,420°C (2,588°F) |
Solubility in Water | Soluble (34.4 g/100 mL at 20°C) |
Density | 1.98 g/cm³ (solid) |
Crystal Structure | Face-centered cubic (FCC) |
Molecular Weight | 74.55 g/mol |
KCl dissolves in water, separating into potassium (K⁺) and chloride (Cl⁻) ions, making the solution conduct electricity.
Equation: KCl (solid) → K⁺ (in water) + Cl⁻ (in water).
When mixed with silver nitrate (AgNO₃), KCl forms white silver chloride (AgCl) and potassium nitrate (KNO₃)
Equation: KCl + AgNO₃ → AgCl (solid) + KNO₃.
Electrolysis of a KCl solution produces potassium hydroxide (KOH), chlorine gas (Cl₂), and hydrogen gas (H₂)
Equation: 2KCl + 2H₂O (electrolysis) → 2KOH + Cl₂ + H₂.
KCl reacts with sulfuric acid (H₂SO₄) to create hydrochloric acid (HCl) and potassium sulfate (K₂SO₄)
Equation: 2KCl + H₂SO₄ → 2HCl + K₂SO₄.
Property | Identifier |
|---|---|
CAS Registry Number | 7447-40-7 |
PubChem Compound ID | 4873 |
PubChem Substance ID | 24852090 |
SMILES Identifier | [Cl-].[K+] |
InChI Identifier | InChI=1/ClH.K/h1H;/q;+1/p-1/fCl.K/h1h;/q-1;m |
InChI Key | WCUXLLCKKVVCTQ-UHVCZDMOCF |
RTECS Number | TS8050000 |
MDL Number | MFCD00011360 |
Property | Rating |
|---|---|
NFPA Health Rating | 1 |
NFPA Fire Rating | 0 |
NFPA Reactivity Rating | 0 |

KCl is widely utilized as a potassium fertilizer in agriculture. Potassium is a crucial nutrient for plant growth, helping in water regulation, the synthesis of proteins, and the activation of enzymes.
In the medical field, KCl is used in various treatments. It’s an essential ingredient in electrolyte replacement solutions and is used to treat hypokalemia, a condition caused by low levels of potassium in the blood. In some cases, it’s used in lethal injections.
As a food additive, KCl serves as a salt substitute to reduce sodium intake, labeled as E508. Potassium chloride (KCl) is utilized in low-sodium and “salt-free” products, assisting individuals in managing blood pressure and promoting heart health.
KCl is utilized in the manufacturing of different chemicals like potassium hydroxide (KOH) and potassium metal. Moreover, it is employed in metal electroplating due to its conductivity.
KCl solutions are employed in laboratories as calibration standards and are extensively utilized in scientific research, especially within the realm of molecular biology. In this field, KCl aids in protein extraction and solubilization, playing a crucial role in various experiments and analyses.
KCl is an ingredient in some types of fire extinguishers, specifically in class potassium extinguishers used for cooking oil or fat fires.
KCl is a significant source of potassium, an essential nutrient that supports heart health, muscle function, and nerve conduction. Using KCl as a supplement can help maintain optimal potassium levels in the body.
As a salt substitute, KCl allows individuals to lower their sodium intake without sacrificing taste. This can be particularly beneficial for people with high blood pressure, heart disease, or kidney problems.
Potassium chloride (KCl) serves as a fertilizer, supplying potassium to enhance crop yield and quality. Potassium helps plants resist drought, disease, and pests, and supports overall plant health.
Potassium chloride (KCl) plays a vital role in treating and preventing potassium deficiency, medically termed hypokalemia. Moreover, it is included in specific medications and treatments, offering crucial support in healthcare settings
Potassium chloride (KCl) finds utility in the manufacturing of various chemicals, including potassium hydroxide and potassium metal. Its properties also make it useful in metal electroplating and as a flux in welding.
Potassium chloride (KCl) solutions serve as essential calibration standards for laboratory instruments in scientific investigations. It’s also crucial in molecular biology for protein studies and DNA extraction processes.
Consuming KCl, especially in supplement form, can lead to gastrointestinal discomfort. This includes symptoms like nausea, vomiting, diarrhea, and abdominal pain.
Some people may experience an unpleasant taste in their mouth after consuming KCl, particularly when used as a salt substitute.
While potassium chloride (KCl) serves as a remedy for potassium deficiency, its improper administration may result in hyperkalemia, an ailment marked by an excess of potassium in the bloodstream. Symptoms of hyperkalemia include muscle weakness, fatigue, irregular heart rhythms, and in severe cases, cardiac arrest.
Individuals with kidney problems need to be cautious with their potassium intake, including KCl, as their kidneys may not be able to remove excess potassium efficiently, leading to an increased risk of hyperkalemia.
While uncommon, certain individuals may experience an allergic reaction to KCl, which can result in symptoms such as itching, rash, or difficulty breathing.
Potassium chloride is beneficial for maintaining electrolyte balance and nerve function, but moderation is key due to potential side effects.
Take potassium chloride as prescribed by a healthcare provider, usually for electrolyte imbalances or low potassium levels.
Fatigue
Muscle Weakness
Cramps
Arrhythmia
Digestive Issues
Muscle Aches
Tingling Sensations
Breathing Difficulties
Mood Changes
Irregular Heartbeat
Low potassium can cause muscle aches, cramps, and weakness, significantly impacting overall physical well-being.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of Potassium Chloride?
KCl
KClO3
KClO4
KClO2
What is the primary use of Potassium Chloride in agriculture?
Pesticide
Fertilizer
Soil conditioner
Herbicide
What is the molar mass of Potassium Chloride (KCl)?
39.1 g/mol
74.6 g/mol
85.0 g/mol
122.5 g/mol
What is the appearance of Potassium Chloride in its pure form?
White crystalline solid
Yellow powder
Colorless liquid
Black crystalline solid
Potassium Chloride is often used in medicine for what purpose?
Antibiotic
Electrolyte replenisher
Pain reliever
Antifungal agent
Which of the following industries uses Potassium Chloride in significant quantities?
Textile
Metal refining
Food processing
Electronics
Potassium Chloride is naturally found in which mineral?
Hematite
Sylvite
Bauxite
Galena
Which property of Potassium Chloride makes it useful in de-icing applications?
Low solubility in water
High melting point
Ability to lower the freezing point of water
Non-corrosive nature
What is the solubility of Potassium Chloride in water at 20°C?
1 g/L
5 g/L
20 g/L
340 g/L
Potassium Chloride is used in which of the following medical treatments?
Chemotherapy
Dialysis
Cardiac arrest
Anesthesia
Before you leave, take our quick quiz to enhance your learning!

