What is the chemical formula of Potassium Hydroxide?
KOH
KCl
K₂O
KO₂


Potassium hydroxide, often known as KOH, is a strong, inorganic compound that plays a crucial role in various scientific and industrial applications. This white solid is highly alkaline and is commonly referred to as caustic potash. It’s widely used in the production of soaps, detergents, and cleaning agents because of its ability to effectively break down organic materials. In the laboratory, potassium hydroxide is essential for chemical analysis and pH control. Due to its corrosive nature, it is important to handle KOH with care, ensuring proper safety measures such as wearing gloves and goggles are in place. This compound is not only fundamental in manufacturing processes but also in environmental management and agriculture, where it helps regulate acidity levels in soils and water.
| Property | Value |
|---|---|
| Formula | KOH |
| Hill Formula | HKO |
| Name | Potassium hydroxide |
| Alternate Names | Caustic Potash, Kaliumhydroxid, Potassa, Potassium Hydrate |
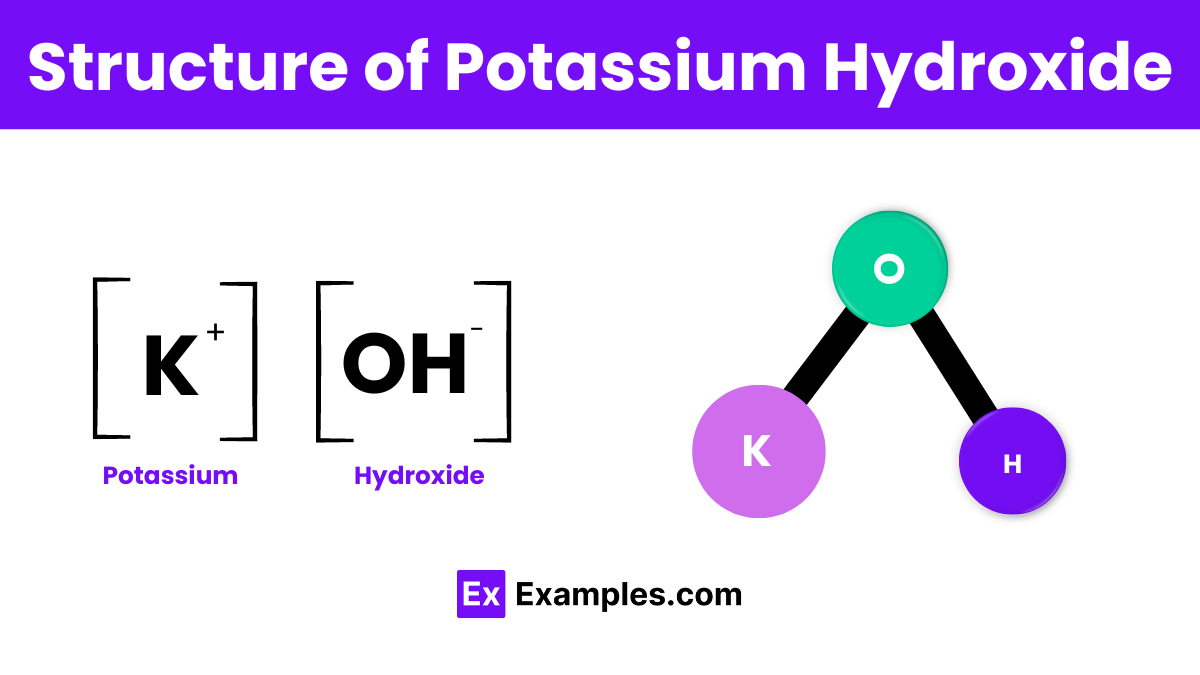
Potassium hydroxide is represented by the chemical formula KOH and it has a simple ionic structure consisting of potassium ions (K⁺) and hydroxide ions (OH⁻). In its solid form, these ions are arranged in a crystal lattice, which is a highly organized structure that helps stabilize the compound. When dissolved in water, potassium hydroxide breaks apart into its constituent ions, which makes it a strong base capable of conducting electricity. This ionic dissolution is crucial for its many uses in chemical reactions and industrial processes, such as soap making and as an electrolyte in batteries.
Potassium hydroxide (KOH) is commonly prepared through the electrolysis of potassium chloride (KCl) solution, a process that involves passing an electric current through the solution to cause a chemical reaction. During this process, potassium chloride, which is salt-like and dissolves well in water, breaks down into potassium ions (K⁺) and chloride ions (Cl⁻). The potassium ions move towards the negative electrode (cathode) where they gain electrons to form potassium metal. Simultaneously, at the positive electrode (anode), water molecules lose electrons and produce hydroxide ions (OH⁻) and hydrogen gas (H₂).
The chemical equation for this reaction is:
In this equation, KCl and H₂O react to form KOH, along with chlorine gas (Cl₂) and hydrogen gas (H₂) as byproducts. The resulting Potassium Hydroxide solution is often concentrated by evaporating the water, leaving behind the solid KOH. This method is highly efficient and widely used in industrial settings to produce potassium hydroxide, which serves various purposes from manufacturing soaps and detergents to serving as an electrolyte in batteries.
| Property | Description |
|---|---|
| Appearance | White or slightly yellow solid with a lumpy or flaky texture |
| Odor | It is typically odorless, making it distinct in this respect |
| Solubility | Highly soluble in water, forming a solution called lye |
| Melting Point | About 360°C (680°F), indicating its stability under heat |
| Boiling Point | Around 1320°C (2408°F), very high, showing it can withstand extreme temperatures |
| Density | Approximately 2.04 g/cm³, making it denser than water |
| Conductivity | Good conductor of electricity when dissolved in water |
| Corrosiveness | Highly corrosive, especially to metals and living tissues |
| Property | Description |
|---|---|
| CAS registry number | 1310-58-3 |
| PubChem compound ID | 6093213 |
| SMILES identifier | [OH-].[K+] |
| InChI identifier | InChI=1/K.H2O/h;1H2/q+1;/p-1/fK.HO/h;1h/qm;-1 |
| EU number | 215-181-3 |
| Gmelin number | 19033 |
| RTECS number | TT2100000 |
| Property | Rating |
|---|---|
| NFPA health rating | 3 |
| NFPA fire rating | 0 |
| NFPA reactivity rating | 1 |
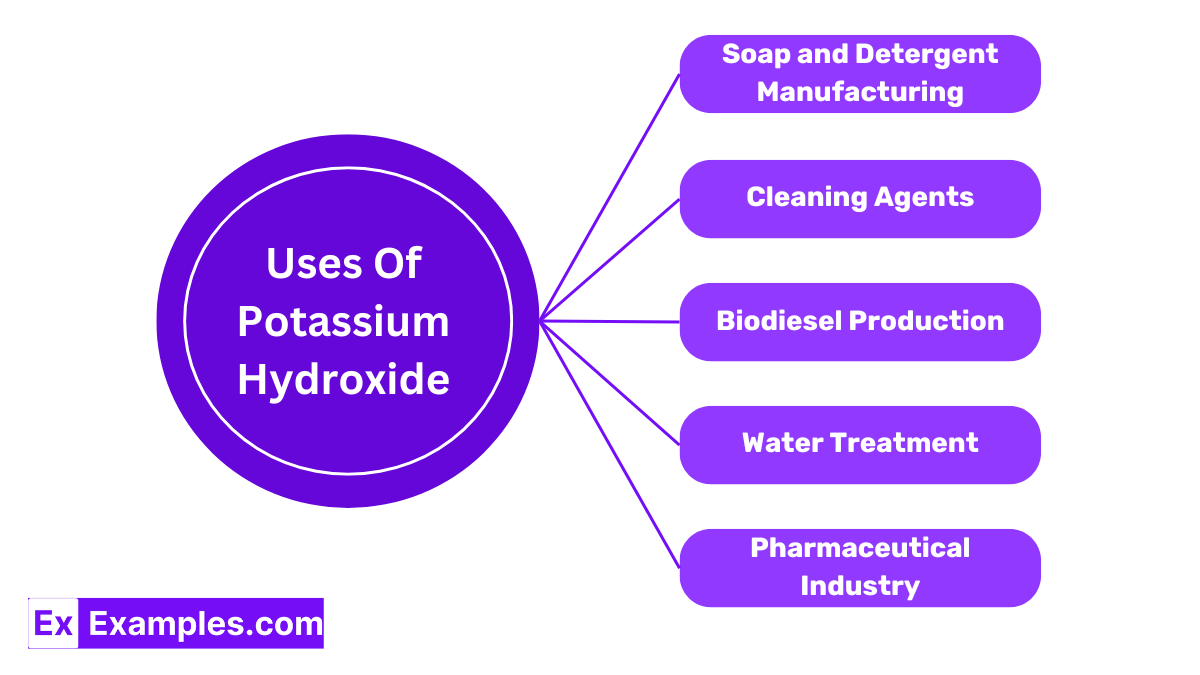
Potassium Hydroxide is crucial in making soft soaps and liquid detergents. Its ability to react with fats and oils in a process called saponification results in soap that is highly effective in cleaning.
KOH is used in the agricultural sector to produce potassium-containing fertilizers. These fertilizers are essential for plant growth and crop yield improvement.
In battery production, Potassium Hydroxide acts as an electrolyte in alkaline batteries. It facilitates the flow of electricity by allowing ions to move freely within the battery.
Potassium Hydroxide adjusts pH levels in various food processing applications. It helps in the preparation of cocoa and chocolate by making them less acidic.
Due to its strong base properties, KOH is used in commercial and industrial cleaners, including drain openers and oven cleaners, to break down organic materials.
In biodiesel industries, potassium hydroxide is used as a catalyst to convert fats and oils into biodiesel through a process called transesterification.
KOH finds applications in the pharmaceutical industry, where it is used to produce soft gels and various drug formulations.
KOH is also employed in water treatment processes to remove hardness and neutralize acidic water.
Yes, Potassium Hydroxide is effective and safe for cleaning when used properly and with appropriate protective measures.
Mixing KOH with water results in an exothermic reaction, releasing heat and forming a solution called lye.
Mixing KOH with vinegar, an acid, produces a neutralization reaction, yielding water and potassium acetate, a type of salt.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of Potassium Hydroxide?
KOH
KCl
K₂O
KO₂
Potassium Hydroxide is commonly known as:
Lye
Baking soda
Vinegar
Table salt
In which of the following industries is Potassium Hydroxide extensively used?
Textile industry
Glass industry
Soap and detergent industry
Petroleum industry
What is the pH of a 1 M solution of Potassium Hydroxide?
0
1
7
14
Which of the following is a common use of Potassium Hydroxide in agriculture?
Fertilizer
pH regulator
Herbicide
Insecticide
What safety precaution is essential when handling Potassium Hydroxide?
Wearing gloves and eye protection
Keeping it away from light
Storing it in the freezer
Handling it with bare hands
Potassium Hydroxide reacts with acids to form:
Salt and water
Salt and hydrogen
Salt and oxygen
Salt and carbon dioxide
What happens when Potassium Hydroxide is dissolved in water?
The solution becomes acidic
The solution becomes basic
The solution becomes neutral
The solution becomes cloudy
Which of the following is a physical property of Potassium Hydroxide?
Yellow color
High melting point
Insoluble in water
Sweet taste
Potassium Hydroxide is used in the production of which type of batteries?
Lithium-ion batteries
Alkaline batteries
Nickel-cadmium batteries
Lead-acid batteries
Before you leave, take our quick quiz to enhance your learning!

