What is the chemical formula of potassium sulfate?
KSO₄
K₂SO₄
K₂SO₃
KSO₃


Potassium sulfate is an inorganic compound commonly used in chemistry. It consists of potassium (K), sulfur (S), and oxygen (O) atoms, forming the chemical formula K₂SO₄. This compound appears as a white, crystalline solid and is highly soluble in water. In the world of compounds, potassium sulfate plays a vital role as a fertilizer, providing essential nutrients that support plant growth. It helps in improving crop yield and quality, making it an important substance in agricultural practices.
| Property | Value |
|---|---|
| Formula | K₂SO₄ |
| Hill formula | K₂O₄S |
| Name | Potassium sulfate |
| IUPAC name | Dipotassium sulfate |
| Alternate names | Dipotassium sulfate |
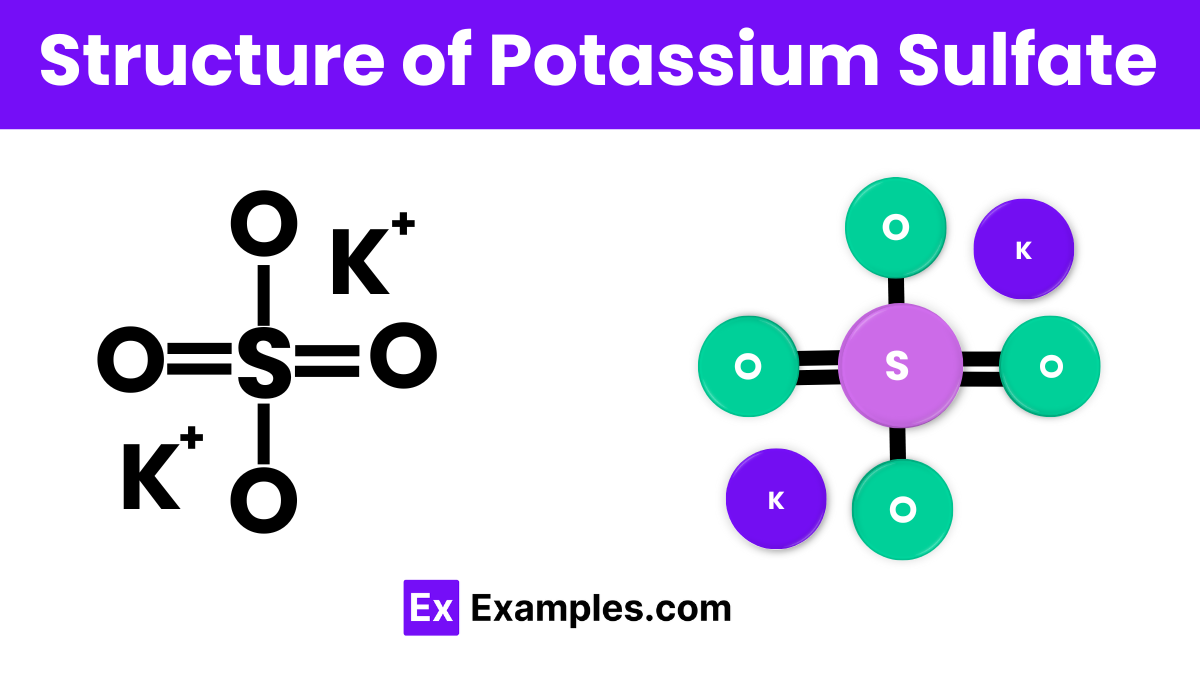
The structure of potassium sulfate (K₂SO₄) consists of two potassium ions (K⁺) and one sulfate ion (SO₄²⁻). In this compound, the sulfate ion forms a tetrahedral shape with one sulfur atom in the center and four oxygen atoms at the corners. The potassium ions are positioned around the sulfate ion, balancing the overall charge. This arrangement allows potassium sulfate to dissolve easily in water, making it effective for use as a fertilizer.
To prepare potassium sulfate (K₂SO₄), you can react potassium chloride (KCl) with sulfuric acid (H₂SO₄). This process is commonly done in a laboratory setting. First, you mix potassium chloride and sulfuric acid in a container. As the reaction occurs, it produces potassium sulfate and hydrochloric acid (HCl) as by-products. The chemical equation for this reaction is:
Another method involves reacting potassium hydroxide (KOH) with sulfuric acid. In this process, you mix potassium hydroxide with sulfuric acid, resulting in the formation of potassium sulfate and water (H₂O). The chemical equation for this reaction is:
Both methods produce potassium sulfate, a valuable compound used mainly as a fertilizer to provide essential nutrients to plants.
| Property | Description |
|---|---|
| Appearance | White crystalline solid |
| Molecular Formula | K₂SO₄ |
| Solubility in Water | Soluble |
| Melting Point | 1,069°C (1,956°F) |
| Density | 2.66 g/cm³ |
| Odor | Odorless |
| Taste | Slightly bitter |
| Property | Value |
|---|---|
| CAS registry number | 7778-80-5 |
| PubChem compound ID | 24507 |
| PubChem substance ID | 24852202 |
| SMILES identifier | [O-]S(=O)(=O)[O-].[K+].[K+] |
| InChI identifier | InChI=1/2K.H2O4S/c;;1-5(2,3)4/h;;(H2,1,2,3,4)/q2*+1;/p-2/f2K.O4S/q2m;-2 |
| RTECS number | TT5900000 |
| MDL number | MFCD00011388 |
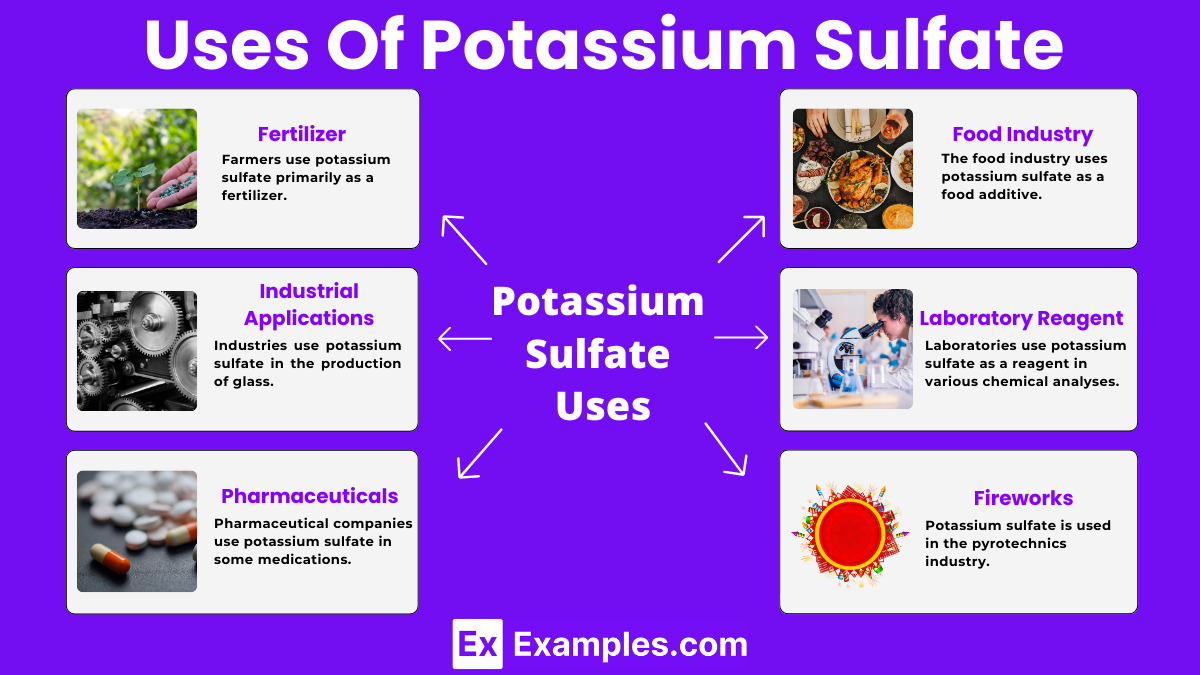
Farmers use potassium sulfate primarily as a fertilizer. It supplies essential nutrients like potassium and sulfur to plants, promoting their growth and increasing crop yields.
Industries use potassium sulfate in the production of glass. It helps improve the strength and clarity of the glass products.
Pharmaceutical companies use potassium sulfate in some medications. It acts as an inactive ingredient or filler in certain tablets and capsules.
The food industry uses potassium sulfate as a food additive. It helps regulate the acidity and provides a source of potassium in some food products.
Laboratories use potassium sulfate as a reagent in various chemical analyses. It helps in experiments and tests requiring a stable, non-reactive substance.
Potassium sulfate is used in the pyrotechnics industry for producing violet-colored flames in fireworks displays. The presence of potassium ions contributes to the characteristic color, making fireworks more vibrant and appealing during celebrations and events.
Potassium sulfate is generally safe for humans in small amounts, but large doses can cause irritation or health issues. Always handle with care and follow safety guidelines.
Potassium sulfate is not safe to drink. Ingesting it can lead to gastrointestinal distress and other health problems. It should be kept away from food and beverages.
Potassium sulfate dissolves in water, releasing potassium and sulfate ions. It increases the water’s nutrient content, making it beneficial for plant irrigation.
Potassium sulfate enriches soil with essential potassium and sulfur, improving plant growth, crop yields, and overall soil fertility. It supports healthy root development and disease resistance.
Potassium sulfate fertilizer can be more expensive than other options. Overuse can lead to soil imbalances and potential runoff issues, negatively impacting the environment.
Plants like fruits, vegetables, and flowering plants benefit from potassium sulfate. It promotes strong growth, improves fruit quality, and enhances resistance to diseases.
Use potassium sulfate during the growing season, especially when plants show signs of potassium deficiency. It is ideal for soil with low sulfur and potassium levels.
Yes, potassium sulfate is good for grass. It strengthens roots, enhances drought resistance, and improves overall lawn health, making it lush and green.
A lawn needs potassium if you see yellowing edges on older leaves, weak growth, and poor resistance to stress. Soil testing can confirm potassium deficiency.
Soil testing is the best way to determine if your lawn needs lime or potassium. Acidic soil needs lime, while potassium-deficient soil requires potassium sulfate.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of potassium sulfate?
KSO₄
K₂SO₄
K₂SO₃
KSO₃
What is the primary use of potassium sulfate in agriculture?
To increase soil acidity
As a nitrogen source
As a potassium source
To improve soil texture
What is the appearance of potassium sulfate in its solid form?
White powder
Yellow crystals
Blue liquid
Red granules
What is the main difference between potassium sulfate and potassium chloride?
Potassium sulfate contains sulfur; potassium chloride does not
Potassium chloride is more soluble in water
Potassium sulfate is less expensive
Potassium chloride has a higher potassium content
How is potassium sulfate typically applied to crops?
As a liquid spray
Through irrigation systems
As a dry granule
Mixed into the soil
Which industry primarily uses potassium sulfate?
Textile industry
Food industry
Agriculture
Pharmaceutical industry
What happens to potassium sulfate when it is dissolved in water?
It forms a yellow solution
It forms a colorless solution
It forms a blue precipitate
It remains undissolved
What is the molecular weight of potassium sulfate (K₂SO₄)?
174.26 g/mol
158.20 g/mol
138.20 g/mol
200.23 g/mol
Which of the following is a common source of potassium sulfate?
Natural mineral deposits
Seaweed
Animal manure
Compost
What is a major benefit of using potassium sulfate over potassium chloride?
Higher potassium content
Additional sulfur content
Lower cost
Better solubility
Before you leave, take our quick quiz to enhance your learning!

