What is the atomic number of Radium?
86
88
90
92
Radium, a luminous chemical element, has long intrigued both scientists and educators. This guide aims to demystify radium, offering teachers a comprehensive understanding of its properties and practical applications. We’ll explore how radium’s unique characteristics can be integrated into educational curricula, sparking students’ curiosity and enhancing their learning experience. With its fascinating history and scientific significance, radium serves as an excellent educational tool, bridging the gap between abstract concepts and tangible examples in the world of science.
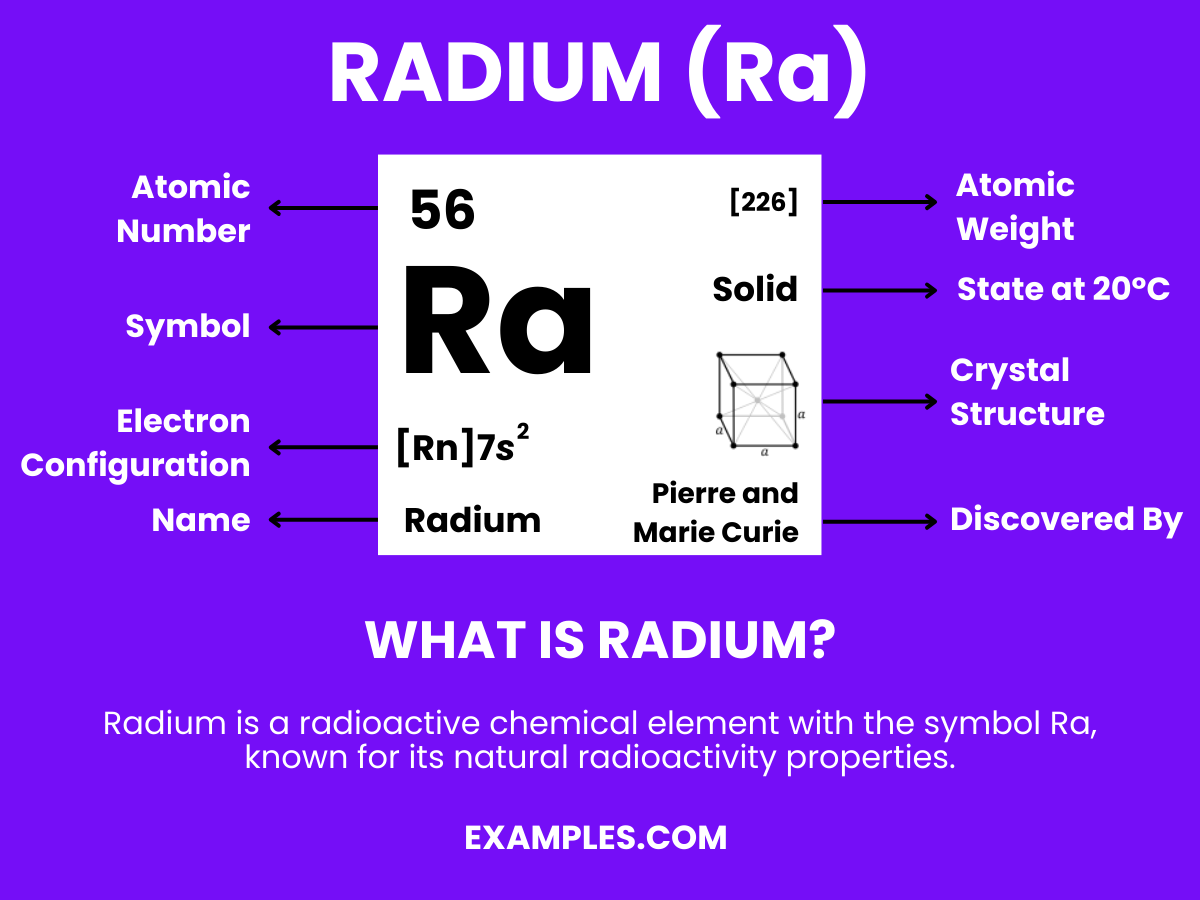
Radium is a radioactive chemical element with the symbol Ra and atomic number 88. It’s a heavy, silvery-white metal, part of the alkaline earth metal group, and is highly radioactive. Discovered by Marie and Pierre Curie in 1898, radium emanates a faint blue glow, a property that has historically fascinated scientists and the public alike. This element decays into radon gas, and its radioactivity is used in various medical and industrial applications. In a classroom setting, radium’s discovery, properties, and uses can serve as a captivating topic, linking chemistry with history and practical science applications.
| Beryllium(Be) |
| Magnesium(Mg) |
| Calcium(Ca) |
| Strontium(Sr) |
| Barium(Ba) |
| Property | Description |
|---|---|
| Appearance | Shiny, silvery-white metal |
| Atomic Mass | 226 u (approximate) |
| Density | About 5 g/cm³ |
| Melting Point | 700°C (1,292°F) |
| Boiling Point | 1,737°C (3,159°F) |
| State at Room Temperature | Solid |
| Radioactivity | Highly radioactive |
| Luminescence | Can glow in the dark due to its radioactivity |
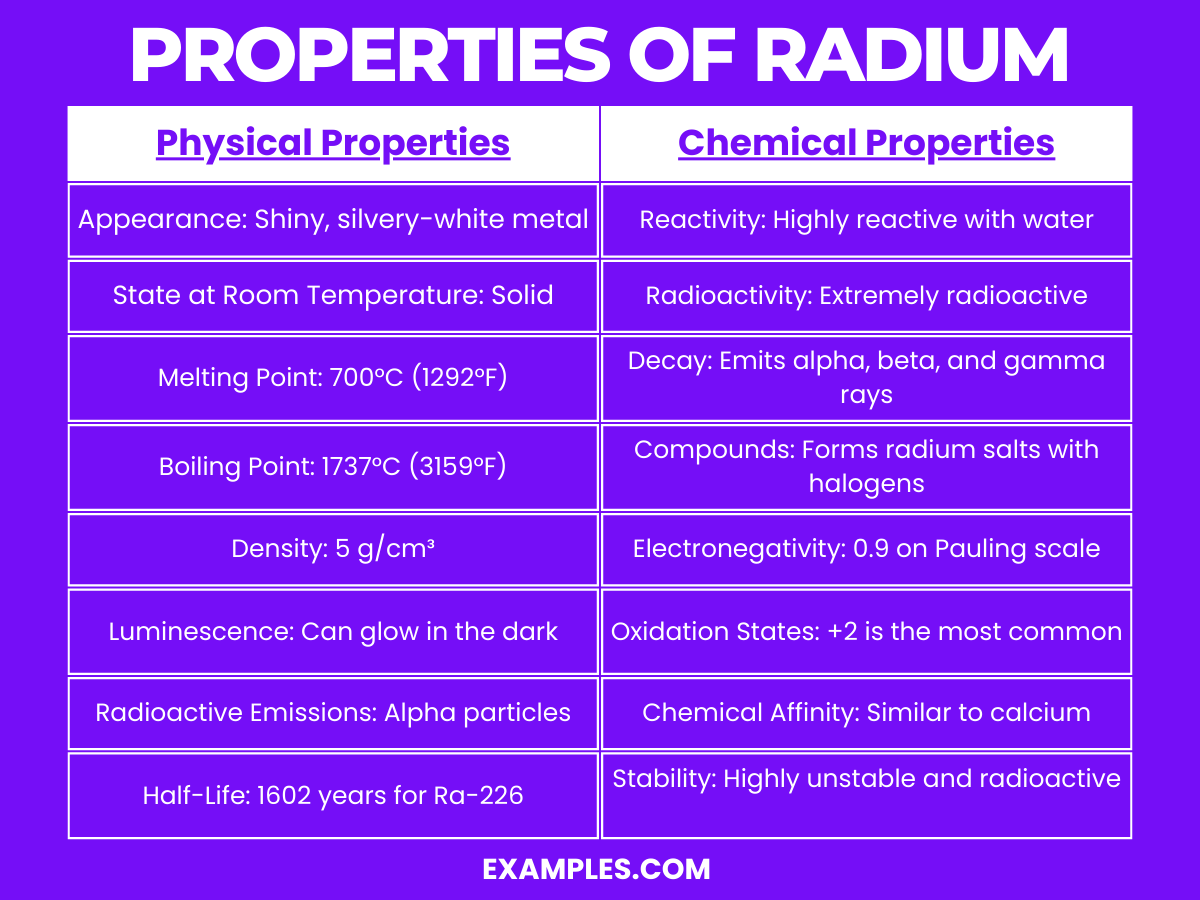
Radium is a highly reactive and radioactive element, categorized under the alkaline earth metals in the periodic table. It exhibits several notable chemical properties:
| Property | Description / Value |
|---|---|
| Melting Point | 700°C (1292°F) |
| Boiling Point | 1737°C (3159°F) |
| Thermal Conductivity | Not well characterized |
| Specific Heat | Not well characterized |
| Heat of Vaporization | Not well characterized |
| Heat of Fusion | Not well characterized |
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | Approx. 5.5 g/cm³ |
| Young’s Modulus | Not well characterized |
| Tensile Strength | Not well characterized |
| Mohs Hardness | Not well characterized |
| Elastic Modulus | Not well characterized |
Note: Due to the rarity and radioactivity of Radium, some material properties are not well characterized or documented.
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Not well characterized |
| Electrical Conductivity | Not well characterized |
Note: Specific electromagnetic properties of Radium are not extensively documented due to its highly radioactive nature.
| Property | Description / Value |
|---|---|
| Atomic Number | 88 |
| Atomic Mass | 226 u (most stable isotope ^226Ra) |
| Neutron Cross Section | Not well characterized |
| Isotopes | Several, including ^223Ra, ^224Ra, ^226Ra, ^228Ra |
| Radioactivity | Highly radioactive; ^226Ra has a half-life of 1600 years |
Radium, a radioactive element, forms several compounds with distinct characteristics. Here are the top six chemical compounds of radium:
Radium has several isotopes, each with unique properties. The table below provides an overview of its notable isotopes:
| Isotope | Symbol | Atomic Mass (u) | Half-Life | Type of Decay |
|---|---|---|---|---|
| Radium-223 | ²²³Ra | 223.0197 | 11.43 days | Alpha decay |
| Radium-224 | ²²⁴Ra | 224.0202 | 3.66 days | Alpha decay |
| Radium-226 | ²²⁶Ra | 226.0254 | 1600 years | Alpha decay |
| Radium-228 | ²²⁸Ra | 228.0311 | 5.75 years | Beta decay |
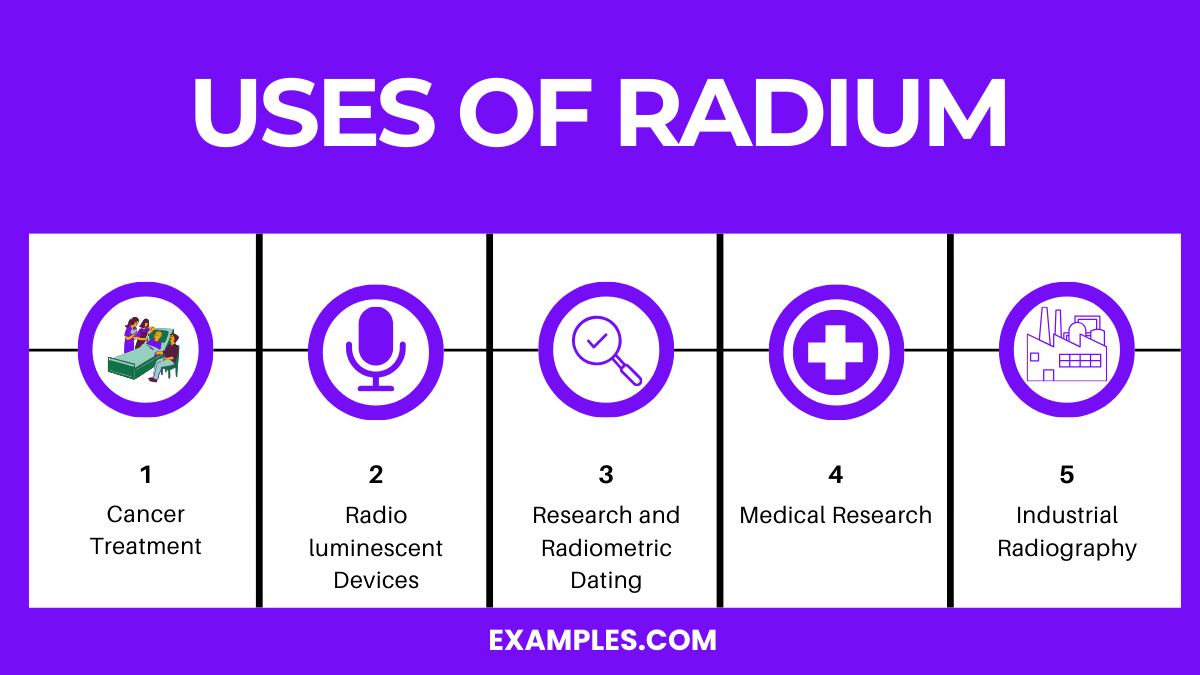
Radium, known for its radioactivity, has several important uses, particularly in medicine and industry. Here are the top five uses:
The commercial production of radium is a complex process, primarily derived from uranium ores, as radium occurs naturally in these ores at low concentrations.
Due to its hazardous nature, the commercial production and use of radium have decreased over time, with safer alternatives being preferred in many applications. However, it remains important in specific medical and industrial contexts.
Radium, a radioactive element, has significant health effects that vary based on exposure level and duration.
The severity of health effects depends on the level and duration of exposure. Modern safety standards and regulations aim to minimize these risks for those who work with radium.
Radium’s impact on the environment is mainly due to its radioactivity and ability to contaminate water and soil.
Managing radium’s environmental impact involves strict regulation of mining and waste disposal, monitoring of water and soil quality, and ensuring that contaminated sites are remediated effectively. These measures are crucial to protect ecosystems and public health from the potential hazards of radium.
Radium is primarily used in cancer treatment, especially for bone metastases, and in scientific research for its radioactive properties.
Radium is not entirely banned in the US, but its use is highly regulated, especially in consumer products, due to its radioactivity.
Yes, radium still exists naturally in small amounts in the Earth’s crust and is also produced artificially for specific medical and research uses.
Radium was historically used in glow-in-the-dark paints, but due to safety concerns, it has been replaced by safer phosphorescent materials.
Radium can cause radiation sickness and increase cancer risk if ingested or inhaled, but it’s also used therapeutically in targeted cancer treatments.
Radium is highly radioactive. It emits alpha particles and can pose significant health risks through prolonged exposure or ingestion.
The primary purpose of radium is in medical treatments, particularly in targeted radiotherapy for cancer, and in scientific research due to its radioactive decay properties.
Radium’s use in medical treatments, particularly in targeted cancer therapies, underscores its significance. While handling this radioactive element requires caution due to health risks, its contributions to science and medicine are invaluable, especially in understanding radioactivity.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
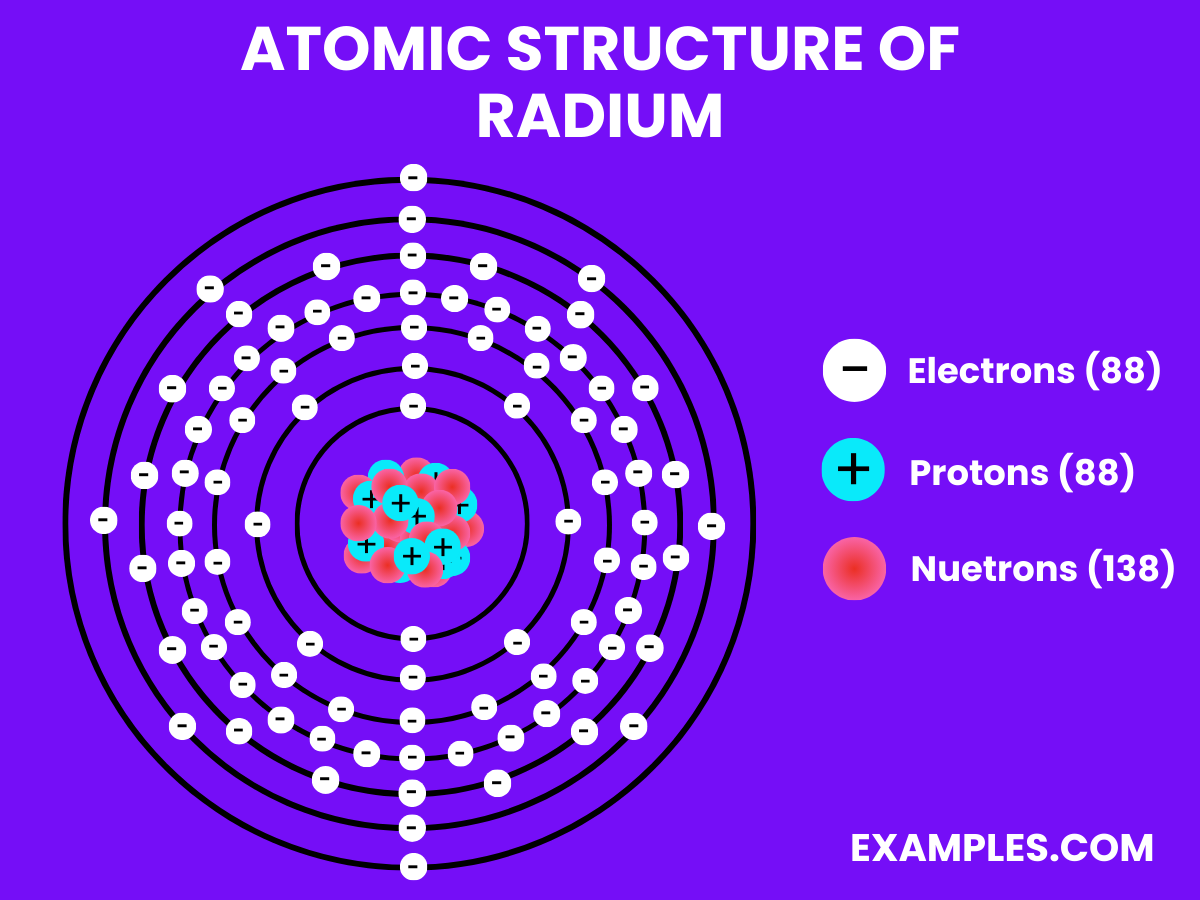
Electrons (88)
Neutrons (138)
Protons (88)
What is the atomic number of Radium?
86
88
90
92
Radium was discovered by which famous scientific duo?
Watson and Crick
Curie and Curie
Hahn and Strassmann
Franklin and Wilkins
Which of the following properties is NOT associated with radium?
Radioactivity
Luminescence
High density
Stability at room temperature
Radium emits which type of radiation?
Alpha particles
Beta particles
Gamma rays
All of the above
What significant medical application did radium have in the early 20th century?
Treating diabetes
Cancer treatment
Vaccine development
Antibiotic production
Radium is a member of which group in the periodic table?
Halogens
Noble gases
Alkaline earth metals
Actinides
What is a common danger associated with radium?
Explosion on contact with water
High flammability
Radioactive decay
Corrosivity
How is radium commonly detected in laboratories?
Litmus test
Geiger counter
Spectrophotometry
pH meter
What happens to radium when it decays?
It becomes lead
It becomes radon
It becomes uranium
It becomes plutonium
In which year was radium first isolated in its pure form?
1898
1902
1910
1923
Before you leave, take our quick quiz to enhance your learning!

