What type of element is Radon?
Metal
Nonmetal
Metalloid
Noble gas
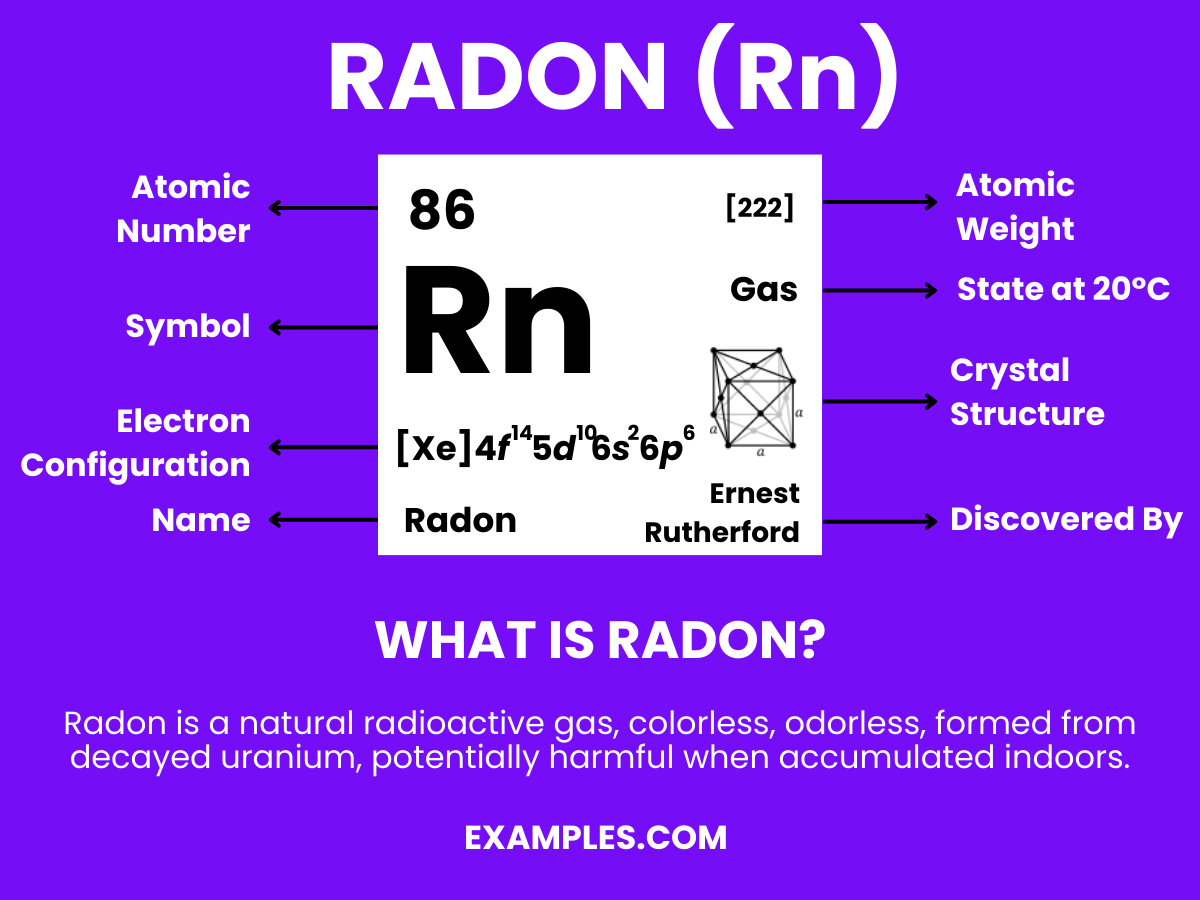
Radon, a natural but elusive element, poses significant interest and challenges in both science and safety. This guide illuminates radon’s definition, characteristics, and its critical role in environmental health. Particularly important for educators, understanding radon is key in fostering awareness and safety in science classes. Dive into the world of radon, equipped with essential knowledge, vivid examples, and practical tips to enrich your teaching experience.
Radon is a colorless, odorless, radioactive gas, occurring naturally as a decay product of uranium. It’s the heaviest gas in the noble gases series and is considered a health hazard due to its radioactivity. Radon is invisible to the naked eye, yet its presence in homes and buildings can pose significant health risks, primarily lung cancer. Understanding its nature and impact is crucial in education to promote awareness and safety measures.
| Helium |
| Neon |
| Argon |
| Krypton |
| Xenon |
Formula: Rn
Composition: A single radon atom.
Bond Type: Typically non-reactive due to a complete valence shell.
Molecular Structure: Monatomic gas.
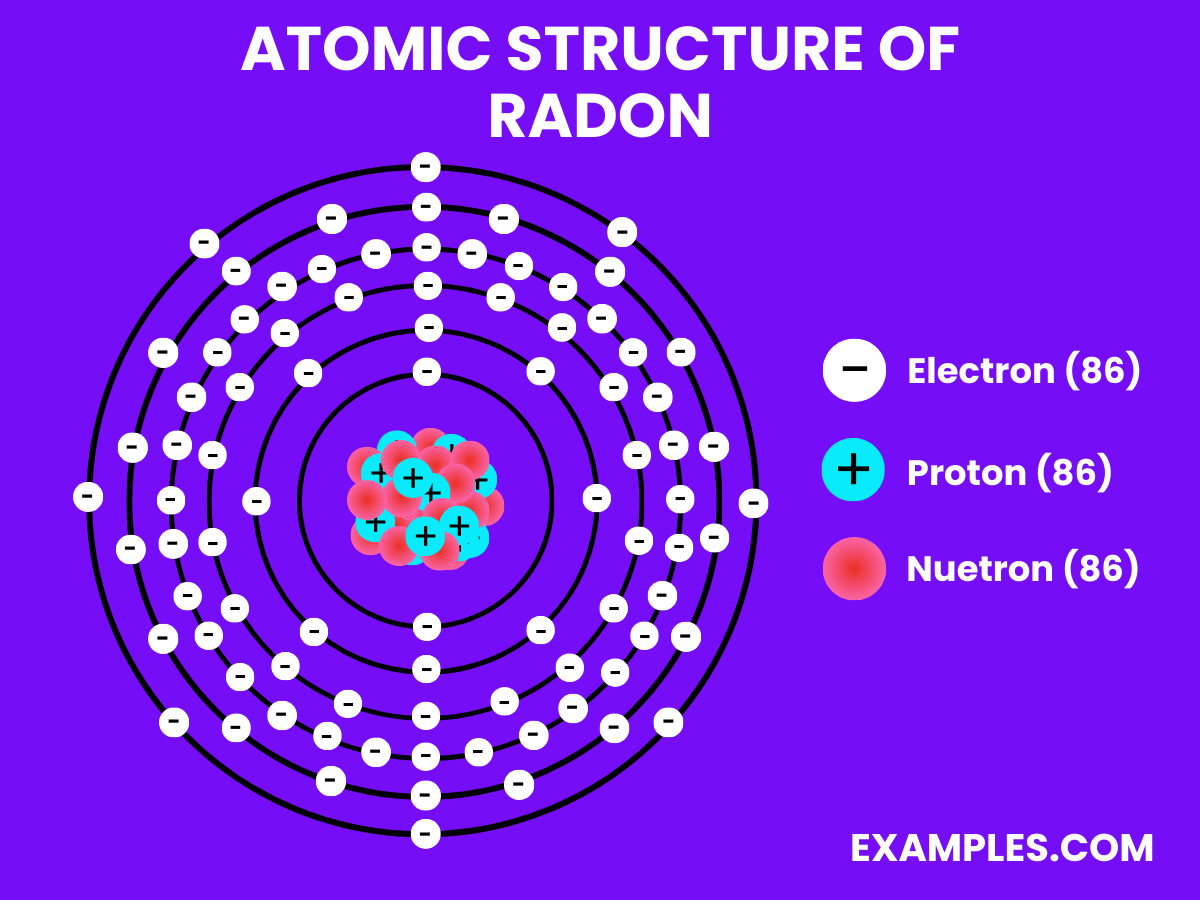
Electron Configuration: 86 electrons; configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 4f¹⁴ 5s² 5p⁶ 5d¹⁰ 6s² 6p⁶.
Significance: Limited use due to radioactivity; research interest in radiology.
Role in Chemistry: Inert, forms few compounds, mainly of academic interest.
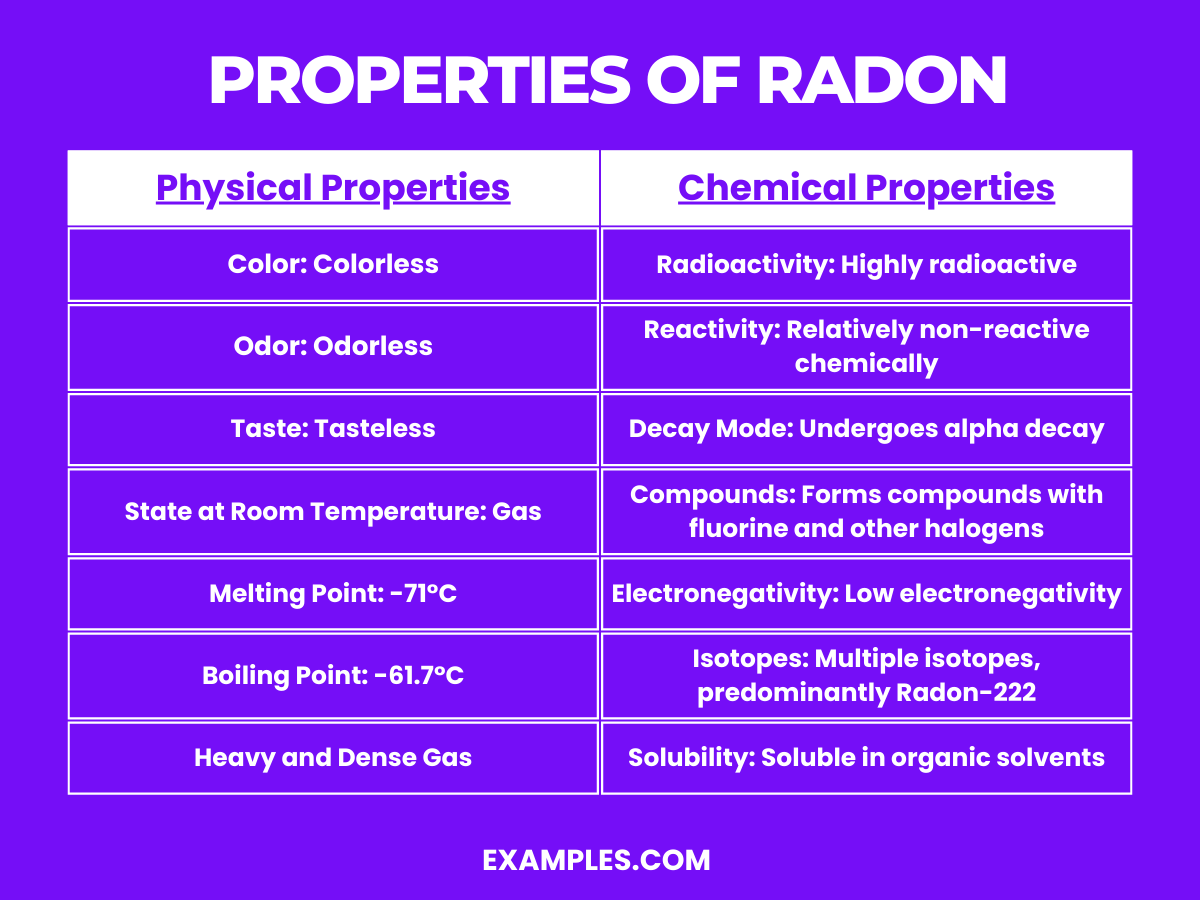
| Physical Property | Description |
|---|---|
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
| State at Room Temperature | Gas |
| Density | 9.73 kg/m³ at standard temperature and pressure (STP) |
| Melting Point | -71°C (-96°F) |
| Boiling Point | -61.7°C (-79°F) |
| Solubility in Water | Low; more soluble in organic solvents |
Radon is a unique element with several interesting chemical properties:
| Property | Description / Value |
|---|---|
| Melting Point | -71°C (-96°F) |
| Boiling Point | -61.7°C (-79.1°F) |
| Thermal Conductivity | 3.61 mW/(m·K) at 0°C |
| Specific Heat | 5.1 J/(mol·K) at 25°C (gas) |
| Heat of Vaporization | 18.10 kJ/mol at boiling point |
| Heat of Fusion | 3.247 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Gas |
| Density | 9.73 kg/m³ at 0°C, 101.325 kPa (gas) |
| Approximately 4.4 g/cm³ at -61°C (liquid) | |
| Solubility in Water | 0.18 cm³/kg at 20°C |
| Color | Colorless (both in gas and liquid forms) |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | Non-conductor (Radon is a noble gas and does not conduct electricity) |
| Property | Description / Value |
|---|---|
| Atomic Number | 86 |
| Atomic Mass | 222 u (most stable isotope, ^222Rn) |
| Neutron Cross Section | 0.7 barns (for ^222Rn) |
| Isotopes | Multiple isotopes, all radioactive |
| Radioactivity | Highly radioactive; ^222Rn (half-life: 3.8235 days), ^220Rn (half-life: 55.6 seconds), among others |
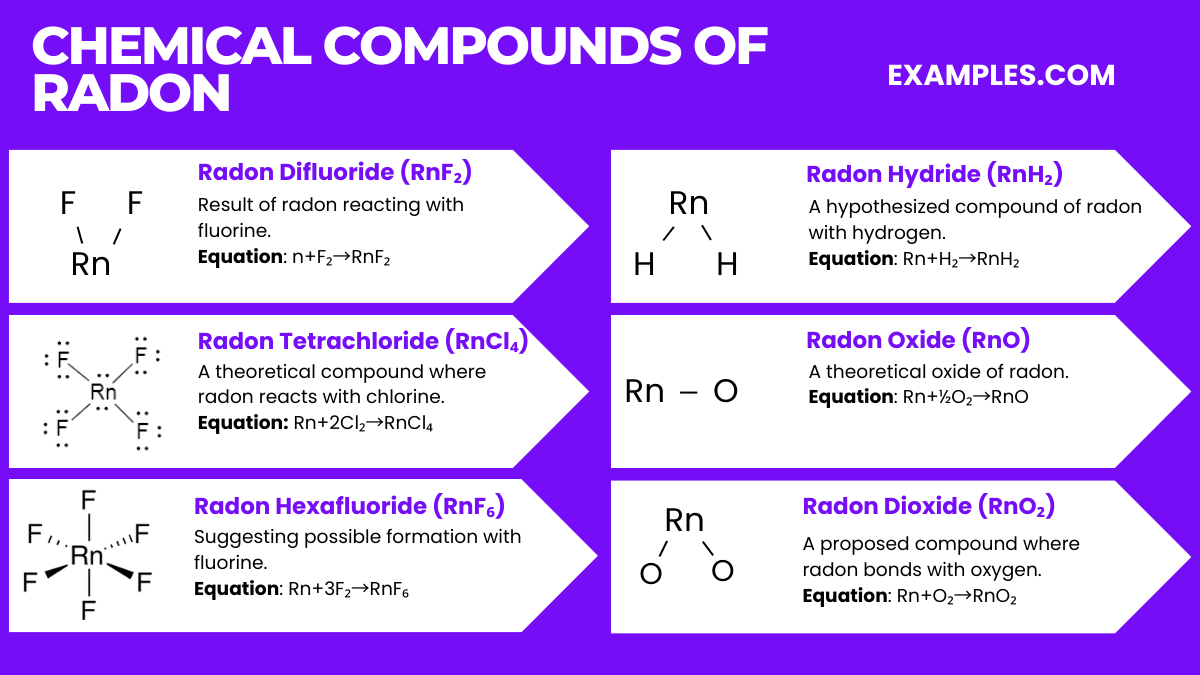
Radon, while generally inert, can form a few compounds under specific conditions. Here are six notable radon compounds along with their relevant chemical equations:
It’s important to note that most of these compounds are theoretical and have not been observed under normal laboratory conditions due to radon’s high radioactivity and short half-life.
Radon has several isotopes, each with unique characteristics. The table below describes some of the key isotopes of radon:
| Isotope | Mass Number | Half-Life | Decay Mode |
|---|---|---|---|
| Rn-219 | 219 | 3.96 seconds | Alpha decay |
| Rn-220 | 220 | 55.6 seconds | Alpha decay |
| Rn-222 | 222 | 3.823 days | Alpha decay |
| Rn-224 | 224 | 1.8 hours | Alpha decay |
| Rn-226 | 226 | 30.9 minutes | Alpha decay |
| Rn-228 | 228 | 65.5 seconds | Alpha decay |
The isotopes of radon are all radioactive, with Radon-222 being the most stable and commonly encountered, especially in environmental and health-related contexts. Their radioactivity primarily involves alpha decay, contributing to radon’s significance in radiological health concerns.
Radon, despite being a radioactive noble gas, has some specialized uses:
Radon is not commercially produced in the same way as many other elements because of its radioactive nature and the health risks associated with its handling. Instead, it is usually obtained as a by-product of the decay of naturally occurring radioactive materials:
Radon is a radioactive gas that poses significant health risks, primarily when it accumulates in indoor environments:
Radon’s environmental effects are primarily related to its radioactivity and how it disperses in the environment:
Radon is used in cancer radiotherapy, geological earthquake prediction, hydrogeological studies, and scientific research due to its radioactivity.
Radon is highly toxic and radioactive, posing serious health risks such as lung cancer, especially when accumulated in indoor spaces.
Radon itself does not glow; however, its radioactive decay can cause other substances to fluoresce, typically not producing visible light.
Yes, radon is a noble gas, characterized by its radioactivity and lack of chemical reactivity under normal conditions.
Radon in homes typically presents no immediate symptoms; long-term exposure increases the risk of lung cancer, without specific radon-related symptoms.
Prolonged exposure to radon can lead to serious health issues, primarily lung cancer, due to its radioactive nature and alpha particle emission.
Radon, a naturally occurring radioactive noble gas, has critical applications in medicine and geology but poses significant health risks, especially lung cancer, upon indoor accumulation. Understanding its properties, ensuring regular home testing, and implementing mitigation strategies are essential for health and safety, highlighting the importance of awareness and responsible handling of radon in various environments.

Radon, a natural but elusive element, poses significant interest and challenges in both science and safety. This guide illuminates radon’s definition, characteristics, and its critical role in environmental health. Particularly important for educators, understanding radon is key in fostering awareness and safety in science classes. Dive into the world of radon, equipped with essential knowledge, vivid examples, and practical tips to enrich your teaching experience.
Radon is a colorless, odorless, radioactive gas, occurring naturally as a decay product of uranium. It’s the heaviest gas in the noble gases series and is considered a health hazard due to its radioactivity. Radon is invisible to the naked eye, yet its presence in homes and buildings can pose significant health risks, primarily lung cancer. Understanding its nature and impact is crucial in education to promote awareness and safety measures.
Formula: Rn
Composition: A single radon atom.
Bond Type: Typically non-reactive due to a complete valence shell.
Molecular Structure: Monatomic gas.
Electron Configuration: 86 electrons; configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 4f¹⁴ 5s² 5p⁶ 5d¹⁰ 6s² 6p⁶.
Significance: Limited use due to radioactivity; research interest in radiology.
Role in Chemistry: Inert, forms few compounds, mainly of academic interest.


Physical Property | Description |
|---|---|
Color | Colorless |
Odor | Odorless |
Taste | Tasteless |
State at Room Temperature | Gas |
Density | 9.73 kg/m³ at standard temperature and pressure (STP) |
Melting Point | -71°C (-96°F) |
Boiling Point | -61.7°C (-79°F) |
Solubility in Water | Low; more soluble in organic solvents |
Radon is a unique element with several interesting chemical properties:
Radioactivity: The most significant chemical property of radon is its radioactivity. Radon is a naturally occurring radioactive gas that is part of the uranium and thorium decay series. It predominantly emits alpha particles during radioactive decay.
Reactivity: Chemically, radon is relatively non-reactive. Being a noble gas, it doesn’t easily form chemical compounds under normal conditions. However, under certain circumstances, particularly involving high energies or the presence of other reactive elements, it can form compounds.
Decay Products: Radon undergoes radioactive decay to form a series of decay products, often referred to as radon progeny or daughters. These are solid, short-lived isotopes like polonium-218, lead-214, and bismuth-214, which are also radioactive.
Compounds: Though rare, radon can form compounds with highly electronegative elements, such as fluorine. Examples include radon difluoride (RnF2). These compounds are generally unstable and of significant interest in research.
Isotopes: Radon has several isotopes, with Radon-222 being the most stable and common. This isotope has a half-life of about 3.8 days and is a significant contributor to environmental radioactivity.
Electronegativity: Radon has a low electronegativity due to its position in the noble gases group in the periodic table. This contributes to its general lack of reactivity.
Solubility: Radon is slightly soluble in water but has a higher solubility in organic solvents. This property is significant in understanding its behavior in different environments, including biological systems.
Property | Description / Value |
|---|---|
Melting Point | -71°C (-96°F) |
Boiling Point | -61.7°C (-79.1°F) |
Thermal Conductivity | 3.61 mW/(m·K) at 0°C |
Specific Heat | 5.1 J/(mol·K) at 25°C (gas) |
Heat of Vaporization | 18.10 kJ/mol at boiling point |
Heat of Fusion | 3.247 kJ/mol at melting point |
Property | Description / Value |
|---|---|
Phase at STP | Gas |
Density | 9.73 kg/m³ at 0°C, 101.325 kPa (gas) |
Approximately 4.4 g/cm³ at -61°C (liquid) | |
Solubility in Water | 0.18 cm³/kg at 20°C |
Color | Colorless (both in gas and liquid forms) |
Property | Description / Value |
|---|---|
Magnetic Susceptibility | Diamagnetic |
Electrical Conductivity | Non-conductor (Radon is a noble gas and does not conduct electricity) |
Property | Description / Value |
|---|---|
Atomic Number | 86 |
Atomic Mass | 222 u (most stable isotope, ^222Rn) |
Neutron Cross Section | 0.7 barns (for ^222Rn) |
Isotopes | Multiple isotopes, all radioactive |
Radioactivity | Highly radioactive; ^222Rn (half-life: 3.8235 days), ^220Rn (half-life: 55.6 seconds), among others |

Radon, while generally inert, can form a few compounds under specific conditions. Here are six notable radon compounds along with their relevant chemical equations:
Radon Difluoride (RnF₂)
Equation: Rn+F₂→RnF₂
Formed under extreme conditions, this compound is a result of radon reacting with fluorine.
Radon Tetrachloride (RnCl₄)
Equation: Rn+2Cl₂→RnCl₄
A theoretical compound where radon reacts with chlorine.
Radon Hexafluoride (RnF₆)
Equation: Rn+3F₂→RnF₆
Another theoretical compound, suggesting possible formation with fluorine.
Radon Hydride (RnH₂)
Equation: Rn+H₂→RnH₂
A hypothesized compound of radon with hydrogen.
Radon Oxide (RnO)
Equation: Rn+½O₂→RnO
A theoretical oxide of radon.
Radon Dioxide (RnO₂)
Equation: Rn+O₂→RnO₂
A proposed compound where radon bonds with oxygen.
It’s important to note that most of these compounds are theoretical and have not been observed under normal laboratory conditions due to radon’s high radioactivity and short half-life.
Radon has several isotopes, each with unique characteristics. The table below describes some of the key isotopes of radon:
Isotope | Mass Number | Half-Life | Decay Mode |
|---|---|---|---|
Rn-219 | 219 | 3.96 seconds | Alpha decay |
Rn-220 | 220 | 55.6 seconds | Alpha decay |
Rn-222 | 222 | 3.823 days | Alpha decay |
Rn-224 | 224 | 1.8 hours | Alpha decay |
Rn-226 | 226 | 30.9 minutes | Alpha decay |
Rn-228 | 228 | 65.5 seconds | Alpha decay |
The isotopes of radon are all radioactive, with Radon-222 being the most stable and commonly encountered, especially in environmental and health-related contexts. Their radioactivity primarily involves alpha decay, contributing to radon’s significance in radiological health concerns.
Radon, despite being a radioactive noble gas, has some specialized uses:
Radiotherapy in Cancer Treatment: Radon is used in some forms of radiotherapy for the treatment of cancer. Radon seeds, containing small amounts of the gas, are placed near or in tumors to deliver targeted radiation therapy, exploiting radon’s alpha-emitting properties.
Earthquake Prediction Research: In geological research, the measurement of radon levels in the ground and water can be an indicator of seismic activity. Elevated radon levels may precede earthquakes, making it a tool for earthquake prediction studies.
Radiation Therapy Research: The unique radioactive properties of radon are utilized in research to develop new radiation therapies for various diseases, especially cancer. Its alpha particles can effectively kill cancer cells with minimal penetration, reducing damage to surrounding healthy tissues.
Hydrogeological Studies: Radon is used in hydrogeological studies to trace the movement of groundwater and gases through soil and rock formations. Its presence helps scientists understand water flow patterns and the origins of water sources.
Climate Change Studies: Radon’s radioactivity and its interaction with atmospheric elements make it useful in climate change research. It helps in studying atmospheric transport, and mixing processes, and provides insights into greenhouse gas emissions.
Radon is not commercially produced in the same way as many other elements because of its radioactive nature and the health risks associated with its handling. Instead, it is usually obtained as a by-product of the decay of naturally occurring radioactive materials:
Extraction from Radium: Radon is commonly derived from the natural decay of radium. Radium compounds, which decay into radon gas, are found in uranium ores. The radon gas is collected from these sources.
Uranium Mining: In uranium mining operations, radon is often released as part of the mining process. It can be collected and isolated for use, especially in research settings.
Laboratory Generation: For research purposes, radon can be generated in laboratories by specifically designed setups that allow the decay of radium-226 to radon-222.
Safety Measures: Due to its radioactivity, the collection, storage, and handling of radon require stringent safety protocols to protect workers and the environment from radiation exposure.
Radon is a radioactive gas that poses significant health risks, primarily when it accumulates in indoor environments:
Lung Cancer: The primary health risk associated with radon exposure is lung cancer. Radon decay products, when inhaled, emit alpha particles that can damage lung tissues and DNA, increasing the risk of lung cancer. This risk is compounded for smokers.
Respiratory Issues: Chronic exposure to high levels of radon can lead to respiratory issues. The radiation can cause damage to lung cells, potentially leading to conditions like emphysema or chronic obstructive pulmonary disease (COPD).
No Immediate Symptoms: Radon exposure does not cause immediate symptoms. The health effects, particularly cancer, may take years to develop, making it a silent health hazard.
Children’s Health: Children are more susceptible to the effects of radon due to their faster breathing rates and developing lungs. Long-term exposure can pose significant health risks for them.
Safety Guidelines and Regulations: Due to its health risks, there are safety guidelines and regulations for acceptable radon levels, especially in residential and occupational settings. Regular monitoring and mitigation measures are recommended in areas with high radon levels.
Radon’s environmental effects are primarily related to its radioactivity and how it disperses in the environment:
Natural Occurrence: Radon is a naturally occurring gas, released from the ground, particularly from areas rich in uranium and radium. It disperses quickly in the outdoor environment, reducing its concentration and impact.
Indoor Accumulation: The primary environmental concern with radon is its accumulation in enclosed spaces like homes, schools, and workplaces. Poor ventilation can lead to high indoor radon levels, posing health risks to occupants.
Soil and Water Contamination: Radon can contaminate groundwater, particularly in areas with certain geological formations. This can lead to indirect exposure through ingestion, although the primary concern remains inhalation of the gas.
Wildlife Impact: While the impact of radon on wildlife is less studied, it’s assumed that animals living in areas with high radon levels could also be at risk, particularly those inhabiting burrows or dens where radon can accumulate.
Ecosystem Impact: The overall impact of radon on ecosystems is considered minimal due to its quick dispersion in the atmosphere and low concentrations outdoors.
Radon is used in cancer radiotherapy, geological earthquake prediction, hydrogeological studies, and scientific research due to its radioactivity.
Radon is highly toxic and radioactive, posing serious health risks such as lung cancer, especially when accumulated in indoor spaces.
Radon itself does not glow; however, its radioactive decay can cause other substances to fluoresce, typically not producing visible light.
Yes, radon is a noble gas, characterized by its radioactivity and lack of chemical reactivity under normal conditions.
Radon in homes typically presents no immediate symptoms; long-term exposure increases the risk of lung cancer, without specific radon-related symptoms.
Prolonged exposure to radon can lead to serious health issues, primarily lung cancer, due to its radioactive nature and alpha particle emission.
Radon, a naturally occurring radioactive noble gas, has critical applications in medicine and geology but poses significant health risks, especially lung cancer, upon indoor accumulation. Understanding its properties, ensuring regular home testing, and implementing mitigation strategies are essential for health and safety, highlighting the importance of awareness and responsible handling of radon in various environments.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What type of element is Radon?
Metal
Nonmetal
Metalloid
Noble gas
Radon is primarily produced by the decay of:
Uranium
Thorium
Radium
Plutonium
Which property of Radon makes it a health hazard?
Flammability
Radioactivity
Toxicity
Reactivity
Radon has the highest concentration in which type of environment?
Open fields
Urban areas
Indoor spaces
Coastal regions
The most common method to test for Radon levels in homes is:
Gas chromatography
Alpha-track detectors
Mass spectrometry
Liquid scintillation
Radon is chemically inert because it:
Has a complete valence shell
Is highly electronegative
Forms stable isotopes
Is a solid at room temperature
Exposure to Radon increases the risk of:
Skin cancer
Lung cancer
Liver cancer
Brain cancer
Radon's atomic number is:
86
88
89
92
To mitigate Radon in buildings, which technique is effective?
Air conditioning
Humidification
Ventilation
Filtration
The stable isotopes of Radon are:
Naturally occurring
Non-existent
Artificially produced
Highly abundant
Before you leave, take our quick quiz to enhance your learning!

