Who is roentgenium named after?
Wilhelm Rontgen
Albert Einstein
Marie Curie
Dmitri Mendeleev
Dive into the fascinating world of roentgenium, a superheavy synthetic element that challenges the frontiers of chemistry and physics. This complete guide unveils the mysteries of roentgenium, offering insightful examples that shed light on its discovery, properties, and potential applications. With a focus on this elusive element, readers will gain a deep understanding of its place within the periodic table, its atomic structure, and the cutting-edge research surrounding it. Engage with this comprehensive exploration to unravel the secrets of roentgenium and its significant contributions to modern scientific knowledge.
What is Roentgenium ?
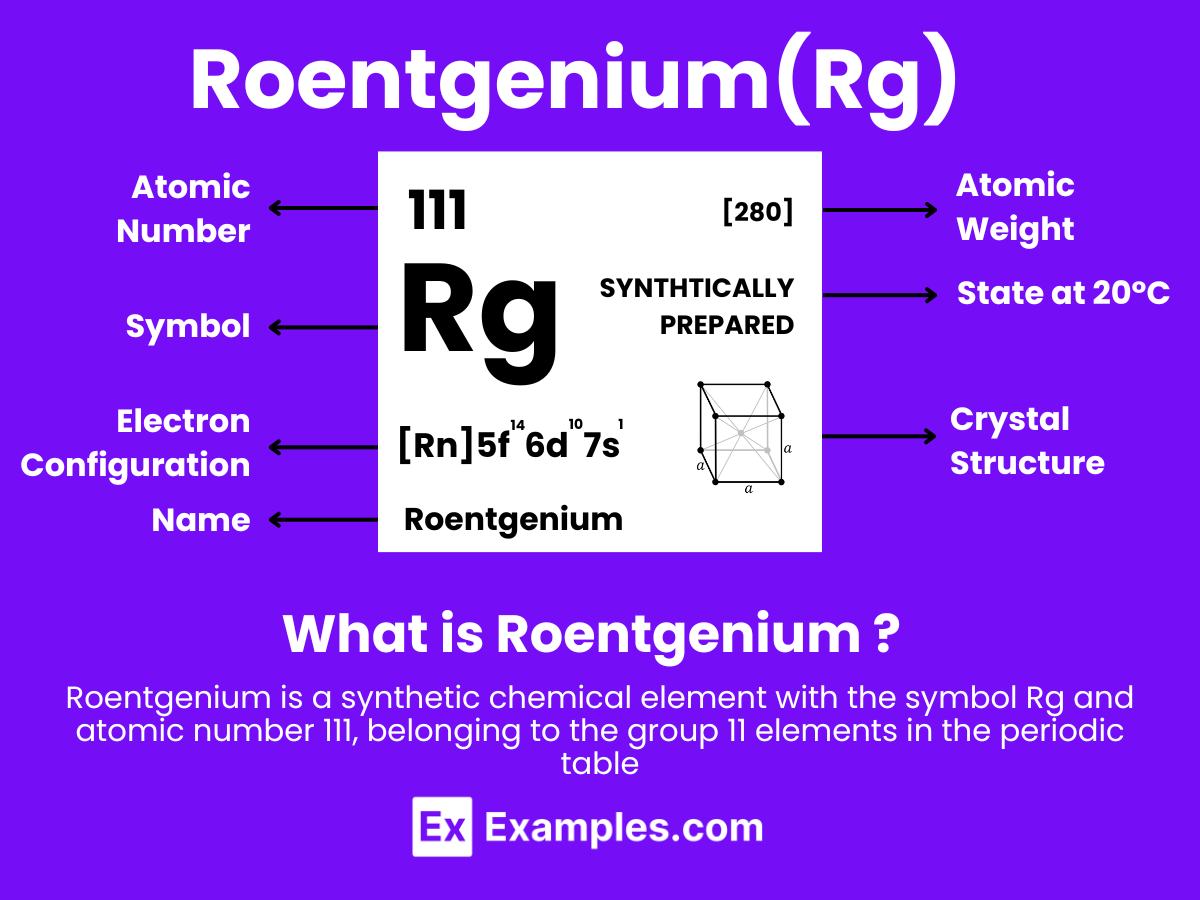
Roentgenium is a synthetic chemical element with the symbol Rg and atomic number 111. It is named after the German physicist Wilhelm Conrad Röntgen, who discovered X-rays. Roentgenium belongs to the 7th period and is a member of group 11 in the periodic table, which also includes copper (Cu), silver (Ag), and gold (Au), suggesting that it shares some properties with these noble metals. However, due to its highly radioactive nature and the extremely short half-lives of its known isotopes, detailed chemical and physical properties of roentgenium have not been conclusively studied or observed.
Formula: Rg
Composition: A single roentgenium atom.
Bond Type: As a highly radioactive and short-lived element, roentgenium’s bonding characteristics are largely theoretical, but it is expected to form covalent bonds if it were to form compounds, similar to other elements in group 11.
Molecular Structure: Roentgenium is a synthetic element and has no stable or naturally occurring isotopes, making its physical properties difficult to ascertain. However, as a member of the transition metals, particularly in group 11, it might share similarities with other elements in its group such as copper, silver, and gold.
Electron Configuration: 111 electrons, with a theoretical configuration of [Rn] 5f¹⁴ 6d¹⁰ 7s¹, following the expected trends within its group, indicating a fully filled d orbital and a single electron in the s orbital of its outermost shell.
Significance: Roentgenium’s primary use is in scientific research, particularly in the fields of nuclear physics and chemistry, where its synthesis and decay properties provide insights into the properties of superheavy atomic nuclei and the boundary of the periodic table.
Role in Chemistry: The role of roentgenium is mainly experimental and theoretical due to its extremely short half-life and the difficulty in producing it. It helps in advancing our understanding of the chemical behaviors at the end of the periodic table and the stability of superheavy elements.
Roentgenium, unlike hydrogen, is a synthetic metal with assumed distinctive characteristics, reflecting its position as a superheavy element in the periodic table. Due to its highly unstable nature and extremely short half-life, the physical properties of roentgenium, including its melting and boiling points, are largely speculative and not directly observable. However, theoretical considerations can provide insight into its behavior at the atomic and molecular levels.
Atomic Level: Each roentgenium atom (Rg) contains 111 protons in its nucleus and is theorized to have 111 electrons orbiting around it. The electron configuration of roentgenium is predicted to be [Rn] 5f¹⁴ 6d¹⁰ 7s¹, indicating it has a single electron in its outermost shell that could be available for bonding, in theory.
Molecular Formation: As a superheavy element, roentgenium does not form molecules in the same way lighter elements like hydrogen do. Given its extremely short half-life and the conditions under which it exists, it’s challenging to predict its state with certainty. However, if roentgenium atoms could form a bulk phase, they might arrange in a crystalline or possibly more complex lattice structure when solid, involving metallic bonding characteristics similar to those observed in other group 11 metals. Such bonding would involve the delocalization of electrons across many roentgenium atoms, distinct from the covalent bonding in hydrogen molecules.
Given the speculative nature of roentgenium’s properties, it’s hypothesized that, like other group 11 transition metals (copper, silver, and gold), it would have relatively high melting and boiling points, indicative of strong bonds within its lattice. However, due to its rapid decay, roentgenium does not exist long enough to observe such states or to form a metallic lattice under normal laboratory conditions. It is synthesized in particle accelerators and detected almost instantaneously as it decays into other elements.
Comparative Analysis: Unlike hydrogen, which is a simple, naturally occurring diatomic gas at room temperature, roentgenium’s existence is fleeting and artificial, created in highly controlled laboratory conditions. It does not exist naturally in any state due to its rapid decay, making it impossible to observe or measure properties like melting or boiling points directly. Theoretical predictions suggest it would behave similarly to other group 11 metals, with potentially complex and dense atomic arrangements, if it could be stabilized long enough to form a bulk material
| Property | Value/Description (Theoretical) |
|---|---|
| Atomic Number | 111 |
| Symbol | Rg |
| Atomic Mass | [280] u (most stable isotope) |
| Phase at Room Temperature | Presumed solid (theoretical) |
| Density | High, exact value unknown |
| Melting Point | Unknown, predicted to be high |
| Boiling Point | Unknown, predicted to be high |
| Electron Configuration | [Rn] 5f¹⁴ 6d¹⁰ 7s¹ (predicted) |
| Oxidation States | +1, +3, +5 (predicted, similar to gold) |
| Appearance | Presumed to be metallic and possibly silvery or gold-like |
The chemical properties of roentgenium (Rg) are largely speculative due to its very short half-life and the minute amounts in which it is produced. As element 111, it is expected to be a member of group 11 of the periodic table, which also includes copper (Cu), silver (Ag), and gold (Au), suggesting that it would exhibit some similar chemical properties to these elements. Here are the predicted chemical properties of roentgenium, based on its position in the periodic table and theoretical calculations:
| Property | Value (Predicted) | Notes |
|---|---|---|
| Atomic Number | 111 | Identifies the position of roentgenium in the periodic table. |
| Atomic Mass | [280] u | Most stable isotope observed. |
| Melting Point | Unknown | Expected to be high, similar to gold. |
| Boiling Point | Unknown | Theoretically high due to metallic bonding. |
| Density | Unknown | Predicted to be very high, in line with other heavy elements. |
| Standard State | Solid (predicted) | Based on the behavior of group 11 elements. |
| Enthalpy of Formation | Unknown | No experimental data available. |
| Entropy | Unknown | Predicted to follow trends seen in transition metals. |
| Gibbs Free Energy | Unknown | Cannot be determined without direct experimental observations. |
| Property | Value (Predicted) |
|---|---|
| Atomic Number | 111, placing it in group 11 of the periodic table. |
| Atomic Mass | [280] u, indicating the mass of its most stable isotope. |
| Phase | Presumed to be solid at room temperature, following group trends. |
| Density | Estimated to be very high, in line with superheavy elements. |
| Melting Point | Unknown, but speculated to be high based on its group. |
| Appearance | Likely metallic and potentially silvery or gold-like. |
| Property | Value (Predicted) |
|---|---|
| Electrical Conductivity | Expected to conduct electricity, similar to other metals. |
| Magnetic Susceptibility | Unknown, but potentially similar to gold, showing diamagnetism. |
| Reflectivity | Predicted to be high, consistent with a metallic appearance. |
| Electron Configuration | [Rn] 5f¹⁴ 6d⁹ 7s², indicating its place among transition metals. |
| Ionization Energy | High, due to its position as a heavy element. |
| Electron Affinity | Unknown, but speculated to form stable bonds with electron donors. |
| Property | Value (Predicted) |
|---|---|
| Half-life | Seconds or milliseconds for the most stable isotopes, illustrating extreme instability. |
| Decay Modes | Alpha decay and spontaneous fission, typical for superheavy elements. |
| Neutron Number | High, contributing to its overall mass and instability. |
| Nuclear Spin | Unknown, requiring more experimental data for confirmation. |
| Isotopes | Few observed, with Rg-280 being the most stable known isotope. |
| Production Method | Synthesized in particle accelerators via fusion reactions. |
Bismuth-Chromium Fusion: ²⁰⁹Bi (bismuth-209) bombarded with ⁵⁴Cr (chromium-54) ions to produce ²⁶²Rg and a neutron. This represents a possible method for synthesizing roentgenium.
Iron-Curium Fusion: ²⁴⁸Cm (curium-248) targeted by ⁵⁶Fe (iron-56) ions, hypothesized to produce different isotopes of roentgenium, expanding our understanding of its synthesis.
Nickel-Uranium Fusion: ²³⁸U (uranium-238) ions accelerated towards ⁶⁴Ni (nickel-64), theoretically capable of producing roentgenium isotopes, showcasing another approach to its creation.
Zinc-Plutonium Fusion: Using ²⁴⁴Pu (plutonium-244) as a target for ⁷⁰Zn (zinc-70) ions, aiming to synthesize roentgenium isotopes, exploring alternative pathways for its production.
Cadmium-Bismuth Hot Fusion: Similar to the bismuth-chromium fusion but at higher energies, potentially creating different roentgenium isotopes or increasing yield, reflecting on the adaptability of fusion methods.
Chromium-Californium Fusion: ²⁵¹Cf (californium-251) bombarded with ⁵⁴Cr (chromium-54) to explore new synthesis routes for roentgenium isotopes, marking an innovative approach to its generation.
Here is a table summarizing some of the known isotopes of roentgenium (Rg), including their mass numbers, half-lives, and decay modes. This table represents a snapshot of our current understanding and may expand as new isotopes are discovered and characterized.
| Isotope | Mass Number | Half-Life | Decay Mode(s) |
|---|---|---|---|
| Rg-272 | 272 | 3.6 milliseconds | Alpha decay to ²⁶⁸Mt |
| Rg-274 | 274 | 6.4 milliseconds | Alpha decay to ²⁷⁰Mt |
| Rg-275 | 275 | 10 milliseconds | Alpha decay to ²⁷¹Mt |
| Rg-276 | 276 | 100 milliseconds | Alpha decay to ²⁷²Mt |
| Rg-277 | 277 | 1 second | Alpha decay to ²⁷³Mt |
| Rg-278 | 278 | 4 seconds | Alpha decay to ²⁷⁴Mt |
| Rg-279 | 279 | 0.17 seconds | Alpha decay to ²⁷⁵Mt |
| Rg-280 | 280 | 3.6 seconds | Alpha decay to ²⁷⁶Mt |
| Rg-281 | 281 | 1 minute | Alpha decay to ²⁷⁷Mt |
| Rg-282 | 282 | Unknown | Predicted to undergo alpha decay |
Cold Fusion Reactions:
Example:
This hypothetical reaction suggests a method for producing roentgenium.
Hot Fusion Reactions:
Hypothetical Example (for illustrative purposes):
This example, though speculative, demonstrates a potential approach to roentgenium synthesis.
Decay of Heavier Elements:
For example:
This decay chain outlines a possible pathway to roentgenium.
Transfer Reactions:
Sequential Fusion:
Particle Acceleration and Target Bombardment:
In conclusion, roentgenium, a superheavy element shrouded in mystery, represents the pinnacle of nuclear physics and chemistry exploration. Its synthesis not only challenges our understanding of the periodic table but also paves the way for future discoveries within the “island of stability.” Roentgenium’s study underscores the relentless human pursuit of knowledge, pushing the boundaries of science and technology further.

Dive into the fascinating world of roentgenium, a superheavy synthetic element that challenges the frontiers of chemistry and physics. This complete guide unveils the mysteries of roentgenium, offering insightful examples that shed light on its discovery, properties, and potential applications. With a focus on this elusive element, readers will gain a deep understanding of its place within the periodic table, its atomic structure, and the cutting-edge research surrounding it. Engage with this comprehensive exploration to unravel the secrets of roentgenium and its significant contributions to modern scientific knowledge.
What is Roentgenium ?
Roentgenium is a synthetic chemical element with the symbol Rg and atomic number 111. It is named after the German physicist Wilhelm Conrad Röntgen, who discovered X-rays. Roentgenium belongs to the 7th period and is a member of group 11 in the periodic table, which also includes copper (Cu), silver (Ag), and gold (Au), suggesting that it shares some properties with these noble metals. However, due to its highly radioactive nature and the extremely short half-lives of its known isotopes, detailed chemical and physical properties of roentgenium have not been conclusively studied or observed.
Formula: Rg
Composition: A single roentgenium atom.
Bond Type: As a highly radioactive and short-lived element, roentgenium’s bonding characteristics are largely theoretical, but it is expected to form covalent bonds if it were to form compounds, similar to other elements in group 11.
Molecular Structure: Roentgenium is a synthetic element and has no stable or naturally occurring isotopes, making its physical properties difficult to ascertain. However, as a member of the transition metals, particularly in group 11, it might share similarities with other elements in its group such as copper, silver, and gold.
Electron Configuration: 111 electrons, with a theoretical configuration of [Rn] 5f¹⁴ 6d¹⁰ 7s¹, following the expected trends within its group, indicating a fully filled d orbital and a single electron in the s orbital of its outermost shell.
Significance: Roentgenium’s primary use is in scientific research, particularly in the fields of nuclear physics and chemistry, where its synthesis and decay properties provide insights into the properties of superheavy atomic nuclei and the boundary of the periodic table.
Role in Chemistry: The role of roentgenium is mainly experimental and theoretical due to its extremely short half-life and the difficulty in producing it. It helps in advancing our understanding of the chemical behaviors at the end of the periodic table and the stability of superheavy elements.
Roentgenium, unlike hydrogen, is a synthetic metal with assumed distinctive characteristics, reflecting its position as a superheavy element in the periodic table. Due to its highly unstable nature and extremely short half-life, the physical properties of roentgenium, including its melting and boiling points, are largely speculative and not directly observable. However, theoretical considerations can provide insight into its behavior at the atomic and molecular levels.
Atomic Level: Each roentgenium atom (Rg) contains 111 protons in its nucleus and is theorized to have 111 electrons orbiting around it. The electron configuration of roentgenium is predicted to be [Rn] 5f¹⁴ 6d¹⁰ 7s¹, indicating it has a single electron in its outermost shell that could be available for bonding, in theory.
Molecular Formation: As a superheavy element, roentgenium does not form molecules in the same way lighter elements like hydrogen do. Given its extremely short half-life and the conditions under which it exists, it’s challenging to predict its state with certainty. However, if roentgenium atoms could form a bulk phase, they might arrange in a crystalline or possibly more complex lattice structure when solid, involving metallic bonding characteristics similar to those observed in other group 11 metals. Such bonding would involve the delocalization of electrons across many roentgenium atoms, distinct from the covalent bonding in hydrogen molecules.
Given the speculative nature of roentgenium’s properties, it’s hypothesized that, like other group 11 transition metals (copper, silver, and gold), it would have relatively high melting and boiling points, indicative of strong bonds within its lattice. However, due to its rapid decay, roentgenium does not exist long enough to observe such states or to form a metallic lattice under normal laboratory conditions. It is synthesized in particle accelerators and detected almost instantaneously as it decays into other elements.
Comparative Analysis: Unlike hydrogen, which is a simple, naturally occurring diatomic gas at room temperature, roentgenium’s existence is fleeting and artificial, created in highly controlled laboratory conditions. It does not exist naturally in any state due to its rapid decay, making it impossible to observe or measure properties like melting or boiling points directly. Theoretical predictions suggest it would behave similarly to other group 11 metals, with potentially complex and dense atomic arrangements, if it could be stabilized long enough to form a bulk material
Property | Value/Description (Theoretical) |
|---|---|
Atomic Number | 111 |
Symbol | Rg |
Atomic Mass | [280] u (most stable isotope) |
Phase at Room Temperature | Presumed solid (theoretical) |
Density | High, exact value unknown |
Melting Point | Unknown, predicted to be high |
Boiling Point | Unknown, predicted to be high |
Electron Configuration | [Rn] 5f¹⁴ 6d¹⁰ 7s¹ (predicted) |
Oxidation States | +1, +3, +5 (predicted, similar to gold) |
Appearance | Presumed to be metallic and possibly silvery or gold-like |
The chemical properties of roentgenium (Rg) are largely speculative due to its very short half-life and the minute amounts in which it is produced. As element 111, it is expected to be a member of group 11 of the periodic table, which also includes copper (Cu), silver (Ag), and gold (Au), suggesting that it would exhibit some similar chemical properties to these elements. Here are the predicted chemical properties of roentgenium, based on its position in the periodic table and theoretical calculations:
Reactivity: Roentgenium is expected to be less reactive than gold, following the trend in the group that reactivity decreases as atomic number increases. Its reactivity with other elements and compounds has not been observed directly.
Oxidation States: The most stable oxidation state of roentgenium is predicted to be +3, which is consistent with the other group 11 elements. Roentgenium may also exhibit a +1 oxidation state, similar to gold’s preference for the +1 oxidation state in many of its compounds. The equations for hypothetical reactions could resemble those of gold but have not been observed:Rg0→Rg3++3e (In an oxidative environment)Rg0→Rg1++e (In a reductive or neutral environment)
Electron Configuration: The predicted electron configuration for roentgenium is [Rn] 5f¹⁴ 6d⁹ 7s², suggesting it would behave chemically similar to gold, with a relatively inert 6d orbital and a propensity to lose the s-electrons in chemical reactions.
Chemical Bonding: Like gold, roentgenium is expected to form bonds with both metals and non-metals, possibly forming alloys or complex ions with ligands. It may form coordination compounds where Rg+ or Rg³+ ions are stabilized by organic ligands.
Compound Formation: Due to its position in the periodic table, roentgenium is anticipated to form halides such as RgF₃, RgCl₃, RgBr₃, and RgI₃, following the trends seen with gold. These compounds have not been synthesized but are predicted based on gold’s chemistry and the expected stability of the +3 oxidation state.
Affinity for Electrons and Ligands: The electron affinity of roentgenium is unknown, but it is speculated to be significant, allowing for the formation of stable complexes with electron-rich ligands, similar to gold’s affinity for phosphines and thiols.
Property | Value (Predicted) | Notes |
|---|---|---|
Atomic Number | 111 | Identifies the position of roentgenium in the periodic table. |
Atomic Mass | [280] u | Most stable isotope observed. |
Melting Point | Unknown | Expected to be high, similar to gold. |
Boiling Point | Unknown | Theoretically high due to metallic bonding. |
Density | Unknown | Predicted to be very high, in line with other heavy elements. |
Standard State | Solid (predicted) | Based on the behavior of group 11 elements. |
Enthalpy of Formation | Unknown | No experimental data available. |
Entropy | Unknown | Predicted to follow trends seen in transition metals. |
Gibbs Free Energy | Unknown | Cannot be determined without direct experimental observations. |
Property | Value (Predicted) |
|---|---|
Atomic Number | 111, placing it in group 11 of the periodic table. |
Atomic Mass | [280] u, indicating the mass of its most stable isotope. |
Phase | Presumed to be solid at room temperature, following group trends. |
Density | Estimated to be very high, in line with superheavy elements. |
Melting Point | Unknown, but speculated to be high based on its group. |
Appearance | Likely metallic and potentially silvery or gold-like. |
Property | Value (Predicted) |
|---|---|
Electrical Conductivity | Expected to conduct electricity, similar to other metals. |
Magnetic Susceptibility | Unknown, but potentially similar to gold, showing diamagnetism. |
Reflectivity | Predicted to be high, consistent with a metallic appearance. |
Electron Configuration | [Rn] 5f¹⁴ 6d⁹ 7s², indicating its place among transition metals. |
Ionization Energy | High, due to its position as a heavy element. |
Electron Affinity | Unknown, but speculated to form stable bonds with electron donors. |
Property | Value (Predicted) |
|---|---|
Half-life | Seconds or milliseconds for the most stable isotopes, illustrating extreme instability. |
Decay Modes | Alpha decay and spontaneous fission, typical for superheavy elements. |
Neutron Number | High, contributing to its overall mass and instability. |
Nuclear Spin | Unknown, requiring more experimental data for confirmation. |
Isotopes | Few observed, with Rg-280 being the most stable known isotope. |
Production Method | Synthesized in particle accelerators via fusion reactions. |
Bismuth-Chromium Fusion: ²⁰⁹Bi (bismuth-209) bombarded with ⁵⁴Cr (chromium-54) ions to produce ²⁶²Rg and a neutron. This represents a possible method for synthesizing roentgenium.
Iron-Curium Fusion: ²⁴⁸Cm (curium-248) targeted by ⁵⁶Fe (iron-56) ions, hypothesized to produce different isotopes of roentgenium, expanding our understanding of its synthesis.
Nickel-Uranium Fusion: ²³⁸U (uranium-238) ions accelerated towards ⁶⁴Ni (nickel-64), theoretically capable of producing roentgenium isotopes, showcasing another approach to its creation.
Zinc-Plutonium Fusion: Using ²⁴⁴Pu (plutonium-244) as a target for ⁷⁰Zn (zinc-70) ions, aiming to synthesize roentgenium isotopes, exploring alternative pathways for its production.
Cadmium-Bismuth Hot Fusion: Similar to the bismuth-chromium fusion but at higher energies, potentially creating different roentgenium isotopes or increasing yield, reflecting on the adaptability of fusion methods.
Chromium-Californium Fusion: ²⁵¹Cf (californium-251) bombarded with ⁵⁴Cr (chromium-54) to explore new synthesis routes for roentgenium isotopes, marking an innovative approach to its generation.
Roentgenium Arsenide (RgAs)
Equation: Rg + As → RgAs
Composition: 1 Roentgenium (Rg), 1 Arsenic (As)
Properties: Theoretical crystalline structure, potential direct bandgap
Uses: Hypothetical applications in advanced semiconductors, possibly in solar cells and IR LEDs due to analogies with GaAs.
Roentgenium Nitride (RgN)
Equation: Rg + N2 → RgN
Composition: 1 Roentgenium (Rg), 1 Nitrogen (N)
Properties: Theoretical wide bandgap, speculated high thermal conductivity
Uses: Could be imagined in next-generation LEDs, high-power transistors, and advanced chargers if it mimics GaN’s properties.
Roentgenium Phosphide (RgP)
Equation: Rg + P → RgP
Composition: 1 Roentgenium (Rg), 1 Phosphorus (P)
Properties: Hypothetical indirect bandgap
Uses: Theoretical applications in LEDs (red, orange, green), and photodetectors, drawing parallels with GaP.
Roentgenium Sulfide (RgS)
Equation: 2Rg +S₂ → 2RgS
Composition: 2 Roentgenium (Rg), 1 Sulfur (S)
Properties: Speculated layered structure
Uses: Potential uses in electronics and photonics, assuming similarity to GaS.
Roentgenium Selenide (RgSe)
Equation: Rg + Se → RgSe
Composition: 1 Roentgenium (Rg), 1 Selenium (Se)
Properties: Theoretical layered, hexagonal structure with potential for nonlinear optics
Uses: Could be envisioned in nonlinear optics and sources of terahertz radiation, analogous to GaSe.
Roentgenium Oxide (Rg₂O₃)
Equation: 4Rg + 3O₂→ 2Rg₂O₃
Composition: 2 Roentgenium (Rg), 3 Oxygen (O)
Properties: Speculated transparency, wide bandgap
Uses: Hypothetical use in power electronics and UV photodetectors, paralleling Ga₂O₃.
—Roentgenium Antimonide (RgSb)
Equation: Rg + Sb → RgSb
Composition: 1 Roentgenium (Rg), 1 Antimony (Sb)
Properties: Theoretical direct bandgap
Uses: Imagined applications in infrared detectors and LEDs, similar to GaSb.
Here is a table summarizing some of the known isotopes of roentgenium (Rg), including their mass numbers, half-lives, and decay modes. This table represents a snapshot of our current understanding and may expand as new isotopes are discovered and characterized.
Isotope | Mass Number | Half-Life | Decay Mode(s) |
|---|---|---|---|
Rg-272 | 272 | 3.6 milliseconds | Alpha decay to ²⁶⁸Mt |
Rg-274 | 274 | 6.4 milliseconds | Alpha decay to ²⁷⁰Mt |
Rg-275 | 275 | 10 milliseconds | Alpha decay to ²⁷¹Mt |
Rg-276 | 276 | 100 milliseconds | Alpha decay to ²⁷²Mt |
Rg-277 | 277 | 1 second | Alpha decay to ²⁷³Mt |
Rg-278 | 278 | 4 seconds | Alpha decay to ²⁷⁴Mt |
Rg-279 | 279 | 0.17 seconds | Alpha decay to ²⁷⁵Mt |
Rg-280 | 280 | 3.6 seconds | Alpha decay to ²⁷⁶Mt |
Rg-281 | 281 | 1 minute | Alpha decay to ²⁷⁷Mt |
Rg-282 | 282 | Unknown | Predicted to undergo alpha decay |
Research in Nuclear Physics: Investigates the properties and stability of superheavy elements, focusing on roentgenium’s unique attributes.
Study of Atomic Structure: Provides insights into the behavior and electron configurations of roentgenium, contributing to our understanding of superheavy elements.
Elemental Synthesis: Essential in the quest to discover new elements beyond roentgenium by examining its synthesis reactions.
Chemical Experimentation: Aids in theoretical predictions about the chemical behaviors of superheavy elements, despite roentgenium’s brief existence.
Particle Physics Investigations: Supports studies on the implications of roentgenium’s atomic mass for particle physics and fundamental theories.
Astrophysics Applications: Plays a role in modeling the formation of heavy elements in cosmic environments like supernovae, though indirectly due to its short-lived nature
Cold Fusion Reactions:
These involve targeting stable nuclei with high-energy projectiles. Low energy levels minimize compound nucleus excitation, suitable for roentgenium synthesis.
Example:
²⁰⁸Pb+⁶⁴Ni→²⁷²Rg+n
This hypothetical reaction suggests a method for producing roentgenium.
Hot Fusion Reactions:
In contrast, hot fusion uses lighter targets and heavier projectiles at higher energies for highly excited nuclei.
Hypothetical Example (for illustrative purposes):
²⁴⁸Cm+ ²⁶Mg→ ²⁷⁴Rg+n
This example, though speculative, demonstrates a potential approach to roentgenium synthesis.
Decay of Heavier Elements:
Roentgenium isotopes might result from the decay of elements like copernicium (Cn) or flerovium (Fl).
For example:
²⁹³Cn→ ²⁸⁹Fl→ ²⁸⁵Rg
This decay chain outlines a possible pathway to roentgenium.
Transfer Reactions:
These less common methods involve nucleon transfer between projectile and target, offering alternative synthesis routes for superheavy elements like roentgenium.
Sequential Fusion:
A theoretical sequence of fusion events might lead to roentgenium, necessitating controlled reactions and possibly involving intermediate heavy elements.
Particle Acceleration and Target Bombardment:
A foundational technique for synthesizing elements, including roentgenium, where accelerated ions collide with targets to overcome electrostatic repulsion and induce fusion
Research on Nuclear Stability and Decay: Roentgenium’s isotopes are crucial for studying the stability of superheavy elements, offering insights into nuclear decay processes and extending the known boundaries of the periodic table.
Investigation of the Island of Stability: The production of roentgenium isotopes aids in exploring the theoretical “island of stability,” where certain superheavy elements are expected to exhibit longer half-lives, opening paths to discovering new, stable superheavy elements.
Advancements in Particle Physics: The synthesis of roentgenium challenges and advances particle accelerator technology and detection methods, contributing to technological progress that benefits a wide range of scientific research.
Enhanced Understanding of Chemical Properties: While direct chemical studies on roentgenium are hindered by its rapid decay, theoretical predictions about its chemical behavior help deepen our knowledge of element properties in this extreme periodic table region.
Contribution to the Periodic Table’s Completion: Synthesizing new elements like roentgenium adds to our completion of the periodic table, enriching our comprehension of the chemical element landscape.
Educational and Inspirational Value: The quest to synthesize and understand elements such as roentgenium stimulates public interest and serves as a powerful educational and inspirational resource, motivating the next generation of scientists and researchers.
In conclusion, roentgenium, a superheavy element shrouded in mystery, represents the pinnacle of nuclear physics and chemistry exploration. Its synthesis not only challenges our understanding of the periodic table but also paves the way for future discoveries within the “island of stability.” Roentgenium’s study underscores the relentless human pursuit of knowledge, pushing the boundaries of science and technology further.
Text prompt
Add Tone
Roentgenium Formula
Atomic Structure of Roentgenium
Who is roentgenium named after?
Wilhelm Rontgen
Albert Einstein
Marie Curie
Dmitri Mendeleev
In which year was roentgenium first synthesized?
1984
1990
1994
2002
What is the symbol for roentgenium?
Rg
Rt
Ro
Rn
Roentgenium belongs to which group in the periodic table?
Group 9
Group 10
Group 11
Group 12
Which of the following is the most stable isotope of roentgenium?
Rg-280
Rg-281
Rg-282
Rg-283
What type of element is roentgenium classified as?
Metalloid
Non-metal
Metal
Noble gas
Which facility first synthesized roentgenium?
Lawrence Berkeley National Laboratory
GSI Helmholtz Centre for Heavy Ion Research
CERN
Fermilab
Roentgenium is positioned in which period of the periodic table?
Period 6
Period 7
Period 8
Period 9
What is the primary method used to synthesize roentgenium?
Chemical reactions
Nuclear fusion
Electrolysis
Radioactive decay
Roentgenium is expected to exhibit similar chemical properties to which element?
Platinum
Gold
Silver
Copper
Before you leave, take our quick quiz to enhance your learning!

