Ruthenium belongs to which group in the periodic table?
Group 7
Group 8
Group 9
Group 10
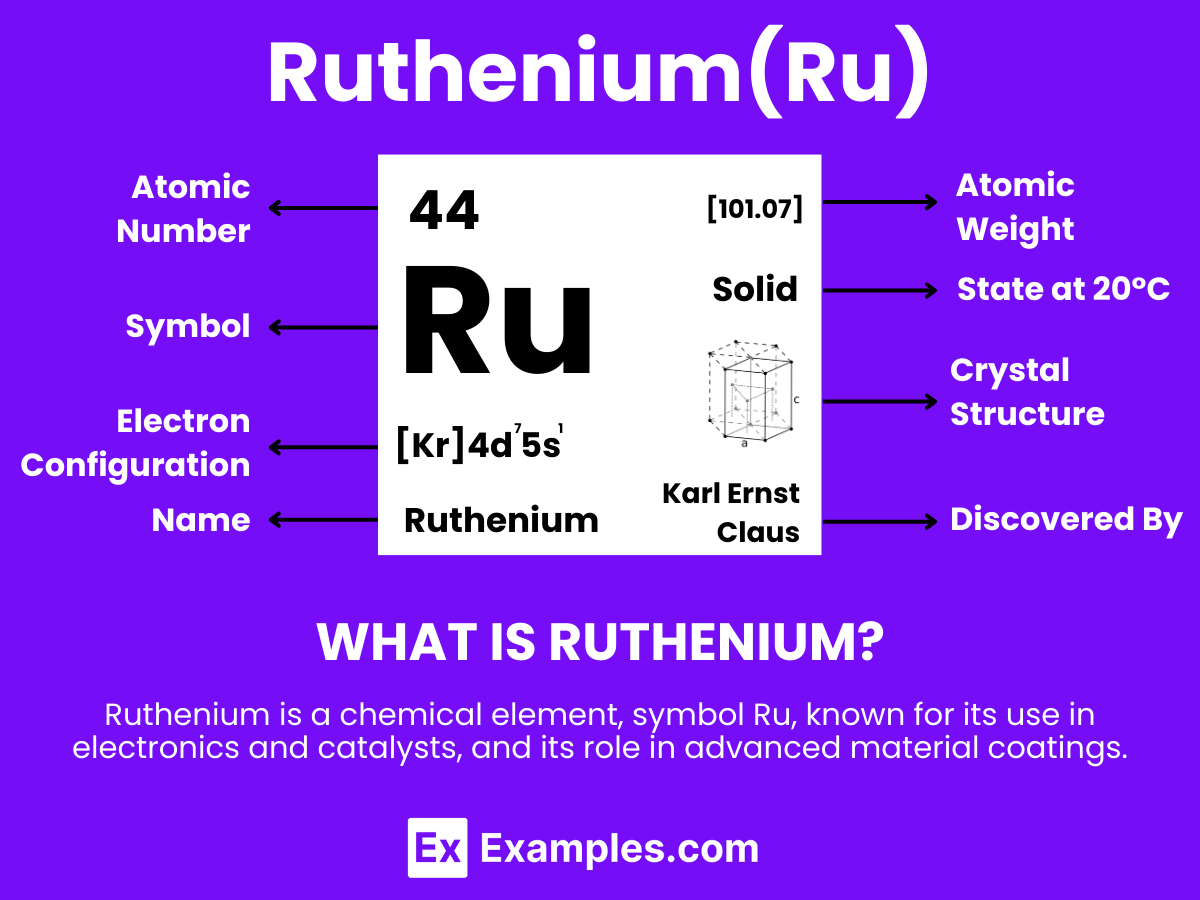
Discover the fascinating world of Ruthenium, a versatile and rare transition metal that plays a pivotal role in modern technology and industrial applications. This complete guide offers an in-depth look into Ruthenium’s unique properties, exploring its various uses from electronics to catalysis. With examples illustrating its significance in enhancing durability and performance, delve into the multifaceted nature of Ruthenium. Uncover how this precious element contributes to advancements in chemical reactions, sustainable energy solutions, and more, making it a key player in the field of advanced materials science.
Ruthenium is a hard, silvery-white metallic element that is distinguished by its remarkable properties and a wide range of applications. With the atomic number 44. Ruthenium is notable for its high resistance to wear and corrosion, making it an ideal candidate for use in harsh environments. This element does not occur freely in nature but is usually found in ores with other platinum group metals from which it is extracted. Ruthenium is Dxtensively used in various fields, particularly in the chemical industry as a catalyst for hydrogenation and ammonia synthesis, and in electronics for manufacturing wear-resistant electrical contacts and thick-film resistors. .
Ruthenium, unlike hydrogen, is a metal with unique characteristics, including a high melting point and an even higher boiling point, showcasing its stability in solid form under standard conditions. Ruthenium’s behavior at the atomic and molecular levels significantly diverges from that of hydrogen, attributable to its position in the periodic table as a transition metal and its metallic nature.
Atomic Level: Each ruthenium atom (Ru) contains 44 protons in its nucleus and has 44 electrons orbiting around it. The electron configuration of ruthenium is ⦏Kr⦐4d⁷5s¹, indicating it has seven electrons in its outermost d shell available for bonding.
Molecular Formation: In its metallic form, ruthenium does not form molecules like H₂. Instead, ruthenium atoms are arranged in a crystalline lattice structure when solid. This structure involves the sharing of electrons between numerous ruthenium atoms in a metallic bond, distinct from the covalent bonding seen in hydrogen molecules. When melted, ruthenium becomes a liquid but retains its metallic bonding characteristics, leading to its significant density and surface tension, even as a liquid.
| Property | Value |
|---|---|
| Atomic Number | 44 |
| Atomic Weight | 101.07 |
| Melting Point | 2,334 °C (4,233 °F) |
| Boiling Point | 4,150 °C (7,502 °F) |
| Density at 20°C | 12.1 g/cm³ |
| State at 20 °C | Solid |
| Color | Silvery-white metallic |
| Electrical Conductivity | 1.45×10⁶ S/m |
| Thermal Conductivity | 117 W/(m·K) |
| Heat of Fusion | 24.06 kJ/mol |
| Heat of Vaporization | 595 kJ/mol |
| Atomic Radius | 134 pm |
| Crystal Structure | Hexagonal close-packed (hcp) |
| Oxidation States | -2, 0, +1, +2, +3, +4, +5, +6, +7, +8 |
Ruthenium, with its symbol Ru and atomic number 44, stands out in the periodic table due to its unique chemical properties. This transition metal is part of the platinum group, exhibiting a rich chemistry and a wide range of oxidation states, from -2 to +8, with +2, +3, and +4 being the most common. Below are some of the key chemical properties of Ruthenium:
| Property | Value |
|---|---|
| Melting Point | 2334 °C |
| Boiling Point | 4150 °C |
| Heat of Fusion | 25.52 kJ/mol |
| Heat of Vaporization | 595 kJ/mol |
| Specific Heat Capacity | 24.06 J/(mol·K) |
| Property | Value |
|---|---|
| Density | 12.45 g/cm³ |
| Mohs Hardness | 6.5 |
| Young’s Modulus | 447 GPa |
| Thermal Conductivity | 117 W/(m·K) |
| Electrical Resistivity | 7.1 µΩ·m (at 20 °C) |
| Property | Value |
|---|---|
| Electrical Conductivity | Moderate, decreases with temperature increase |
| Magnetic Susceptibility | Paramagnetic at room temperature |
| Superconductivity | Does not exhibit superconductivity under normal conditions |
| Property | Value |
|---|---|
| Natural Isotopes | Ru-96, Ru-98, Ru-99, Ru-100, Ru-101, Ru-102, Ru-104 |
| Most Stable Isotope | Ru-106 with a half-life of 373.59 days |
| Neutron Cross Section | Varied, depends on isotope |
| Primary Decay Mode | Beta decay for heavier isotopes |
| Isotope | Natural Abundance (%) | Half-Life | Notes |
|---|---|---|---|
| Ru-96 | 5.54 | Stable | — |
| Ru-98 | 1.87 | Stable | — |
| Ru-99 | 12.76 | Stable | — |
| Ru-100 | 12.60 | Stable | — |
| Ru-101 | 17.06 | Stable | — |
| Ru-102 | 31.55 | Stable | — |
| Ru-104 | 18.62 | Stable | — |
| Ru-106 | Trace | 373.59 days | Used in radiation therapy for eye and skin cancers |
Ruthenium, a versatile element in the platinum group metals, has numerous applications across various industries due to its unique properties:
Ruthenium highlighting its remarkable chemical diversity through six key compounds. Each compound showcases Ruthenium’s versatility in applications ranging from organic synthesis to electrochemical processes and solar energy conversion. Understanding these compounds illuminates Ruthenium’s crucial role in advancing various scientific and industrial fields, underlining its significance beyond its mere position on the periodic table.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Ruthenium belongs to which group in the periodic table?
Group 7
Group 8
Group 9
Group 10
What is the symbol for ruthenium?
Ru
Rn
Re
Rh
Which of the following properties is true about ruthenium?
Highly reactive
Low melting point
Excellent conductor of electricity
Non-metal
What is the primary use of ruthenium in industry?
Jewelry
Catalysts
Batteries
Paints
Ruthenium is commonly found in which type of mineral deposits?
Iron ores
Platinum ores
Copper ores
Aluminum ores
What is the melting point of ruthenium?
1234°C
1663°C
2334°C
2730°C
Ruthenium is a member of which block in the periodic table?
s-block
p-block
d-block
f-block
Which country is one of the largest producers of ruthenium?
China
South Africa
Brazil
India
What is the density of ruthenium?
10.12 g/cm³
12.45 g/cm³
18.12 g/cm³
21.02 g/cm³
What color is ruthenium in its pure form?
Silver-gray
Gold
Black
White
Before you leave, take our quick quiz to enhance your learning!

