What is the chemical formula for table salt?
KCl
NaCl
CaCl2
MgCl2


Salts are chemical compounds that result from the reaction between an acid and a base. They are made up of positively charged ions, known as cations, and negatively charged ions, called anions. These ions are held together by ionic bonds, forming a crystalline structure. Common table salt, or sodium chloride, is a familiar example of a salt. In water, salts typically dissolve into their component ions. They are essential in numerous daily life processes and play pivotal roles in various industries, including food, medicine, and cleaning products. Understanding salts is crucial in chemistry because they help us comprehend how substances interact in both nature and man-made environments.
Sodium chloride is commonly known as table salt, is the most familiar type of salt. It is used primarily in food seasoning and preservation. Chemically, it consists of sodium and chloride ions and is crucial for human body functions, such as nerve transmission and muscle function.
Epsom salt is not actually a salt but a naturally occurring mineral compound of magnesium and sulfate. It is well-known for its use in bath salts to relieve sore muscles. Magnesium sulfate also helps plants absorb phosphorus and nitrogen.
Rock salt, or halite, is another form of sodium chloride similar to table salt but in a larger crystal form. It is mined from salt deposits and is commonly used to melt ice on roads and walkways during winter. It is also used in cooking, particularly for grilling and creating salt crusts on meats.
Iodized salt is regular sodium chloride with a small amount of iodine added. Iodine is essential for thyroid function, and adding it to salt helps prevent iodine deficiency disorders such as goiter, making iodized salt an important health innovation.
Sea salt is produced through the evaporation of seawater. It contains traces of minerals like magnesium, calcium, and potassium, giving it a slightly different flavor and texture than table salt. Sea salt is often used in cooking and cosmetics.
Kosher salt has a larger, flakier crystal than regular table salt, which makes it ideal for koshering, the process of drawing blood out of meat. It’s also favored in cooking for its ease in handling and ability to dissolve quickly.
Himalayan pink salt is rock salt mined in Pakistan. Its pink color comes from trace minerals in the salt, including iron, calcium, and magnesium. This salt is popular in culinary uses for its unique color and claimed health benefits.
Though not a traditional salt, sodium bicarbonate (baking soda) functions similarly in cooking. It’s a leavening agent used in baked goods like bread and cakes. When mixed with an acid, it produces carbon dioxide gas, causing dough to rise.
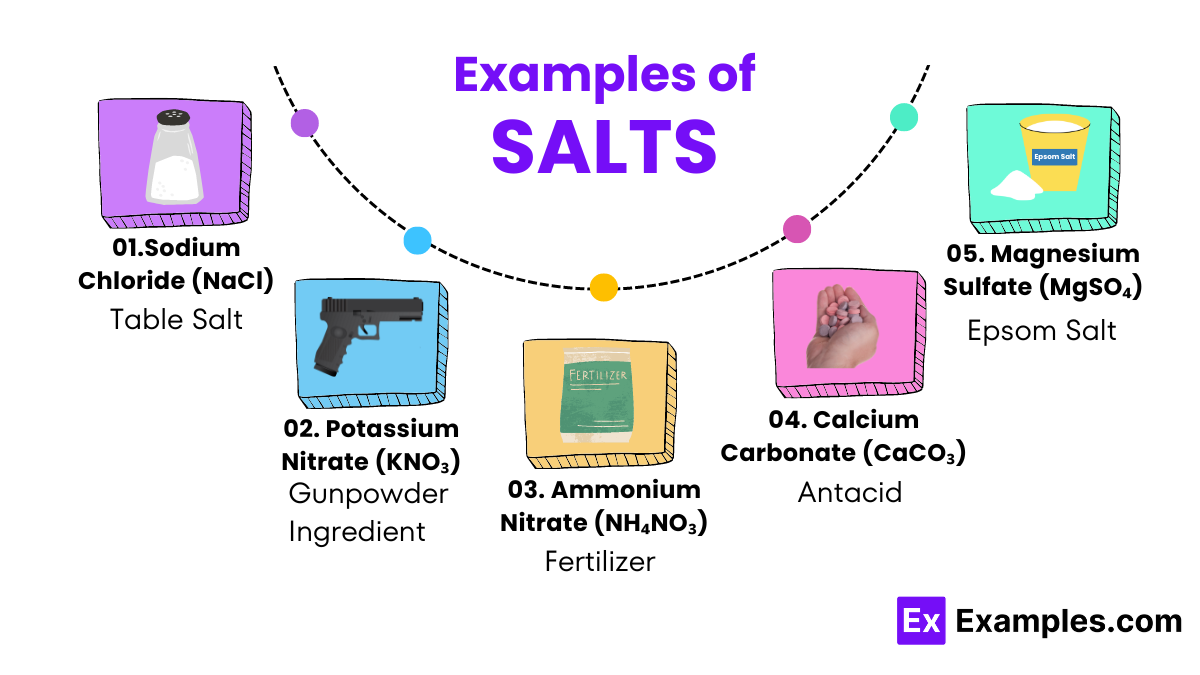
Identifying different types of salts is a fundamental skill in chemistry that involves observing physical properties and conducting simple chemical tests. Salts, which are compounds formed from the reaction between an acid and a base, can be identified by their appearance, solubility, and how they react with other substances. For instance, table salt (sodium chloride) usually appears as small, clear crystals that dissolve easily in water, while Epsom salt (magnesium sulfate) forms larger, distinct crystals that also dissolve but can have a laxative effect. Simple flame tests can further distinguish salts: sodium imparts a bright yellow color to a flame, whereas potassium yields a violet color. Additionally, chemical tests that involve adding acids can reveal carbon dioxide bubbles if the salt contains carbonate ions.
Salts are formed through a chemical process known as neutralization, where an acid reacts with a base to produce a salt and water. This type of reaction is fundamental in chemistry and has practical applications in various industries, including pharmaceuticals and agriculture. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), a base, they form sodium chloride (NaCl), commonly known as table salt, and water (H₂O). The general equation for this reaction is:
In addition to neutralization, salts can also be formed by reacting a metal with an acid, where the metal displaces the hydrogen from the acid, forming a salt and hydrogen gas. For instance, when magnesium (Mg) reacts with hydrochloric acid, magnesium chloride (MgCl₂) and hydrogen gas (H₂) are produced. Understanding these processes helps students appreciate how different substances interact in everyday life, leading to the formation of new compounds with unique properties.
| Property | Description |
|---|---|
| Color | Varies |
| Shape | Crystalline structure |
| Taste | Salty, bitter, or sour |
| Solubility | Generally soluble in water |
| Melting Point | High |
| Conductivity | Conducts electricity when molten or dissolved in water |
Salts can appear in various colors depending on the elements involved. For instance, sodium chloride (table salt) is white, whereas copper sulfate is a vibrant blue. The presence of different metal ions influences the color of the salt.
Salts typically form a crystalline structure, meaning they consist of a repeated arrangement of atoms in a three-dimensional lattice. This structure contributes to the characteristic shapes of salt crystals, which can be seen under a microscope.
The taste of salts can vary significantly. While sodium chloride tastes salty, other salts like potassium nitrate can have a bitter taste, and magnesium sulfate (Epsom salt) has a distinctly sour flavor. The taste is influenced by the metal and nonmetal ions that make up the salt.
Most salts are soluble in water, which means they can dissolve to form a solution. The solubility of a salt depends on its chemical structure and the temperature of the water. For example, sodium chloride dissolves readily in water, making it a good conductor of electricity in solution.
Salts generally have high melting points due to the strong ionic bonds between the cations and anions in their crystalline lattice. This property makes them stable at room temperature and useful in high-temperature processes.
When salts are dissolved in water or melted, they can conduct electricity. This is because the dissolution or melting process allows the ions to move freely, enabling them to carry electrical current. This property is utilized in various applications, including electrolysis and batteries.
Salts are formed through the ionic bonding of cations (positively charged ions) and anions (negatively charged ions).
Most salts exhibit a crystalline structure where ions are arranged in a regular, geometric pattern. This gives salts their characteristic solid form.
The ionic bonds in salts contribute to their high melting and boiling points, making them solid at room temperature.
Salts are generally soluble in water. Their solubility can vary greatly depending on the nature of the salt and the temperature of the water.
When dissolved in water or melted, salts become electrolytes, conducting electricity due to the movement of the free ions.
Salts can influence taste, with the most common being the salty flavor of sodium chloride. However, different salts can have varying tastes, such as bitter or sour.
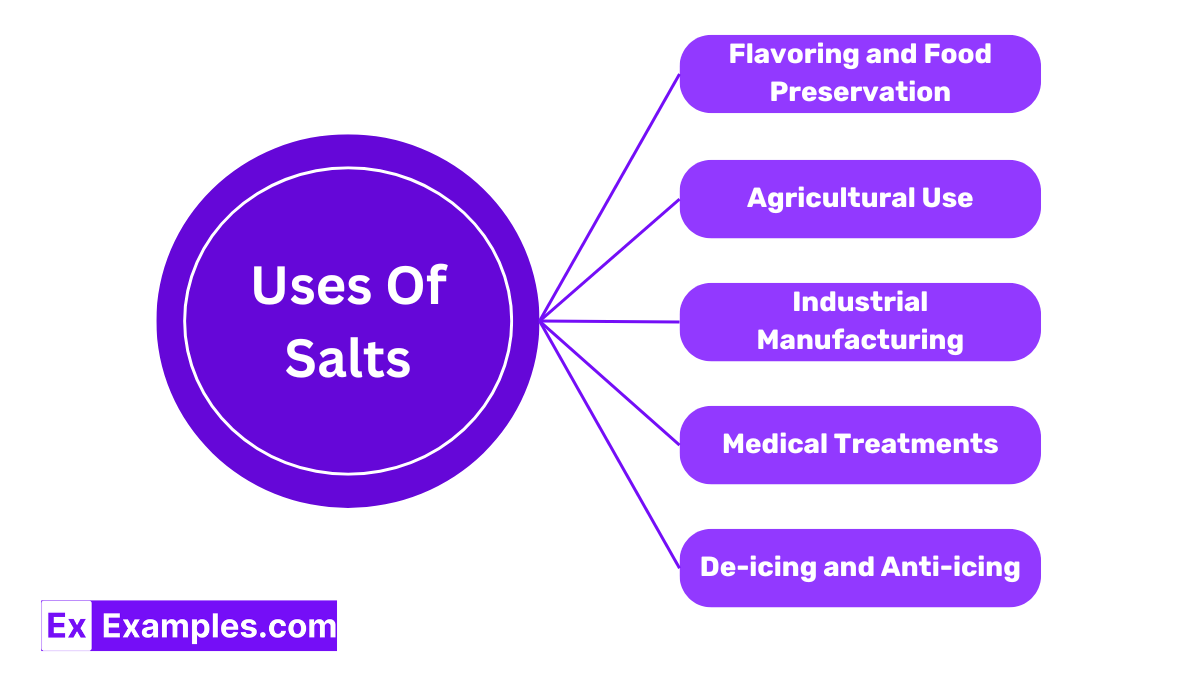
One of the most well-known uses of salt, particularly sodium chloride (table salt), is for seasoning food. Its ability to enhance flavor is unmatched. Salt is also crucial in food preservation, as it inhibits the growth of bacteria, thus extending the shelf life of foods like meats and pickles.
Certain salts, such as potassium nitrate, are used extensively in agriculture. They serve as fertilizers, providing essential nutrients (nitrogen, potassium, and phosphorus) that help plants grow and enhance their ability to withstand diseases and pests.
Salts are integral in various industrial processes. For instance, sodium chloride is used in the manufacture of glass, paper, polyester, and rubber. It is also essential in the production of other chemicals through the process of electrolysis.
Epsom salt (magnesium sulfate) is widely used in the medical field. It can relieve muscle soreness and reduce swelling. It is also used as a laxative to treat occasional constipation.
Hard water, which contains high levels of calcium and magnesium, can damage pipes and appliances. Salts, such as sodium chloride, are used in water softeners to replace these hard ions with sodium ions, softening the water and extending the life of plumbing systems and appliances.
During the winter months, salts like calcium chloride and rock salt are spread on roads and walkways to melt ice and snow. This helps to prevent accidents and ensures safer travel conditions.
Sea salt is often found in health and beauty products. It’s used in bath salts, body scrubs, and facial treatments for its exfoliating properties, which can help to cleanse the skin and promote relaxation.
A rare type of salt that intrigues chefs and health enthusiasts alike is Bischofite. Bischofite is a hydrous magnesium chloride mineral (MgCl2·6H2O) that is much rarer than common salts like sodium chloride. It originates from ancient seabeds and is primarily found in geological formations. Bischofite is particularly noted for its therapeutic properties. It is used in hydrotherapy and balneotherapy for its purported benefits in relieving joint and muscle pain, improving circulation, and reducing inflammation. Its rarity and unique health benefits make Bischofite a sought-after salt among those looking to leverage natural mineral salts for health and wellness applications.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula for table salt?
KCl
NaCl
CaCl2
MgCl2
Which of the following salts is used to prevent tooth decay in toothpaste?
NaF
NaCl
KCl
CaCO₃
What is the pH of a neutral salt solution?
1
14
7
0
What type of reaction typically produces a salt?
Decomposition
Synthesis
Neutralization
Combustion
Which of the following is a property of salts?
Poor solubility in water
High melting and boiling points
Poor electrical conductivity
Low density
What is the role of salts in the human body?
They act as enzymes
They help maintain fluid balance and nerve function
They are a source of energy
They provide structural support to cells
What is the common name for the hydrated salt Na₂SO₄·10H₂O?
Glauber's salt
Epsom salt
Table salt
Baking soda
Which of the following salts is used as a desiccant to absorb moisture?
NaCl
KCl
CaCl₂
NaNO₃
Which of the following salts is commonly used in the agricultural industry as a fertilizer?
NaCl
KNO₃
CaCO₃
MgSO₄
Which salt is commonly known as Glauber's salt?
NaCl
Na₂SO₄·10H₂O
NaHCO₃
NaNO₃
Before you leave, take our quick quiz to enhance your learning!

