What is the chemical formula of silver nitrate?
AgNO₃
AgCl
Ag₂O
Ag₂SO₄

Silver nitrate is a chemical compound with a formula of AgNO₃. It appears as a transparent crystalline solid that is highly soluble in water. In the world of chemistry, silver nitrate plays a crucial role due to its diverse applications, including in photography, in staining glass, and as an antiseptic in healthcare. Its ability to react with other compounds makes it an invaluable substance in various chemical reactions and experiments.
| Formula | AgNO₃ |
| Name | Silver nitrate |
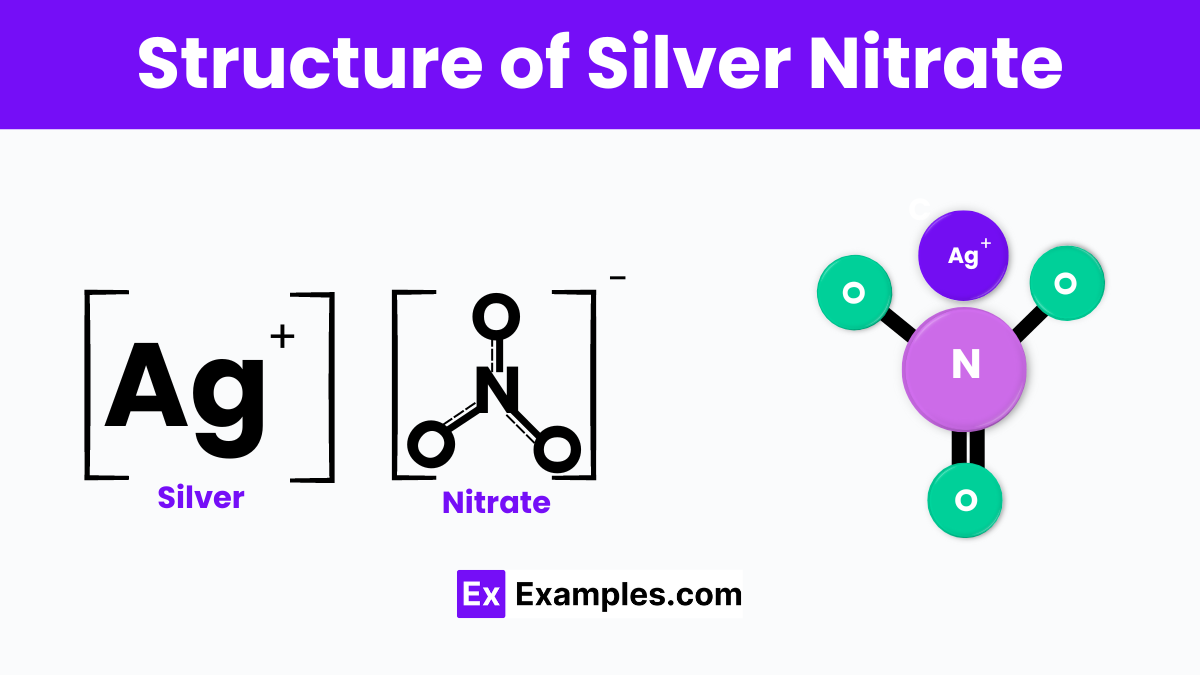
Silver nitrate is a chemical compound with the formula AgNO₃, where Ag represents silver, N is nitrogen, and O₃ stands for three oxygen atoms. It forms as a crystalline solid that is transparent to white in color and is highly soluble in water. At the molecular level, silver nitrate consists of silver ions (Ag⁺) connected to nitrate ions (NO₃⁻). This ionic structure contributes to its ability to dissolve readily in water, as the water molecules interact with and separate the silver and nitrate ions.
In its solid form, silver nitrate adopts a rhombohedral crystal structure, which is a type of crystalline arrangement where the molecules are organized in a repeating pattern. This structure is crucial for its various applications, including in photography, medicine, and as a precursor to other silver-containing compounds. The ionic nature of silver nitrate explains its high reactivity, especially its ability to form precipitates with halide ions, which is utilized in analytical chemistry to detect the presence of halides.
The preparation of silver nitrate typically involves the reaction of silver with nitric acid. This chemical process can be described in two main steps. Initially, pure silver metal is carefully placed into a reaction vessel containing dilute nitric acid. The choice of dilute acid is crucial to ensure a controlled reaction, as concentrated nitric acid can lead to a more vigorous and potentially hazardous process. Upon contact, the silver reacts with the nitric acid to produce silver nitrate (AgNO₃), water (H₂O), and nitrogen dioxide (NO₂) gas as by-products. The chemical equation for this reaction is written as:
The nitrogen dioxide gas released is a toxic brown fume, indicating that the reaction should be carried out in a well-ventilated area or under a fume hood.
After the reaction completes, the resulting mixture contains silver nitrate dissolved in water along with excess nitric acid. The next step involves purifying the solution to obtain pure silver nitrate. This is typically done by evaporating the water from the mixture, which can be achieved through gentle heating. As the water evaporates, silver nitrate begins to crystallize out of the solution. Once all the water has evaporated, the solid silver nitrate can be collected. It is important to store the final product in dark, amber-colored glass containers to protect it from light, as silver nitrate is photosensitive and can decompose upon exposure to light, leading to the formation of silver metal and nitrogen oxides.
| Property | Description |
|---|---|
| Appearance | White crystalline solid |
| Molecular Weight | 169.87 g/mol |
| Melting Point | 209.7 °C (409.5 °F) |
| Boiling Point | Decomposes at 440 °C (824 °F) |
| Density | 4.35 g/cm³ |
| Solubility in Water | Highly soluble; increases with temperature |
| Solubility in Other Solvents | Soluble in ammonia, slightly soluble in ethanol and methanol |
| Refractive Index | 1.744 (solid) |
| Thermal Conductivity | Low |
| Chemical Stability | Stable under ordinary conditions but light-sensitive, decomposes to silver and nitrogen oxides upon exposure to light or high heat |
| Safety Considerations | Corrosive to skin and eyes, potentially toxic if ingested or inhaled, handles with appropriate safety equipment |
Silver nitrate is highly soluble in water, which is a fundamental property for its use in solutions.
Equation: AgNO₃(s)→Ag⁺(aq)+NO₃⁻(aq)
When silver nitrate reacts with halide ions, it forms insoluble silver halides, a property utilized in photographic processes and in the detection of halides.
Equation: AgNO₃(aq)+X⁻(aq)→AgX(s)+NO₃⁻(aq)
Where X⁻ = Cl⁻, Br⁻, or I⁻, leading to the formation of AgCl, AgBr, or AgI, respectively.
In the presence of aldehydes, silver nitrate participates in the Tollens’ test, producing a silver mirror.
Equation: RCHO+2AgNO₃+3NH₃+H₂O→2Ag+RCOONH4+2NH₄NO₃
This equation showcases the reduction of silver ions to metallic silver by the aldehyde.
Can cause burns upon skin contact and requires careful handling.
| Property | Value |
|---|---|
| CAS Registry Number | 7761-88-8 |
| PubChem Compound ID | 24470 |
| PubChem Substance ID | 24852261 |
| SMILES Identifier | N⁺([O⁻])[O⁻].[Ag⁺] |
| InChI Identifier | InChI=1/Ag.NO3/c;2-1(3)4/q+1;-1 |
| RTECS Number | VW4725000 |
| MDL Number | MFCD00003414 |
While silver nitrate is invaluable in various applications, it requires careful handling. It can cause stains on skin and fabrics, which turn black upon exposure to sunlight. Moreover, it is important to note that silver nitrate solutions and solids should be stored away from light to prevent decomposition and maintain efficacy.
Silver nitrate is safe for humans when used correctly, but excessive or improper use can cause skin irritation, eye damage, and argyria.
Silver nitrate can promote wound healing by preventing infection, but it does not directly speed up the healing process of tissues.
Skin stained by silver nitrate typically remains black for 2-3 weeks but can last longer depending on exposure and individual skin reaction.
Silver nitrate is toxic if ingested, inhaled, or contacted with skin, causing burns. It’s harmful to aquatic life, requiring proper disposal, and should be securely stored away from children and incompatible substances.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of silver nitrate?
AgNO₃
AgCl
Ag₂O
Ag₂SO₄
What is the primary use of silver nitrate in medical applications?
Disinfectant
Antiseptic
Analgesic
Anesthetic
Silver nitrate reacts with sodium chloride to form:
Silver sulfate
Silver bromide
Silver chloride
Silver oxide
Silver nitrate solution is often used to test for the presence of which ion?
Chloride ion
Sulfate ion
Phosphate ion
Carbonate ion
What is the molar mass of silver nitrate?
123.3 g/mol
169.9 g/mol
187.8 g/mol
212.8 g/mol
In what type of chemical reaction does silver nitrate typically participate?
Synthesis
Decomposition
Single displacement
Double displacement
What safety precaution is necessary when handling silver nitrate?
Wear insulated gloves
Use in a well-ventilated area
Avoid exposure to sunlight
Wear protective clothing
Silver nitrate is soluble in:
Ethanol
Water
Benzene
Chloroform
The reaction between silver nitrate and potassium iodide produces:
Silver iodide
Silver sulfate
Silver oxide
Silver carbonate
Silver nitrate can be used to determine the presence of which compound in water?
Sulfates
Nitrates
Chlorides
Phosphates
Before you leave, take our quick quiz to enhance your learning!

