What is the atomic number of Silver?
46
47
48
49
Dive into the lustrous world of Silver, a precious metal that captivates with its versatility and brilliance. This comprehensive guide illuminates Silver’s rich history, its pivotal role in various industries, and the myriad of uses it offers, from jewelry making to technological applications. We’ll explore the elemental properties that make Silver so valuable, delve into its fascinating compounds, and showcase real-world examples. Whether for investment, industry, or innovation, Silver’s allure remains unmatched, promising endless possibilities.
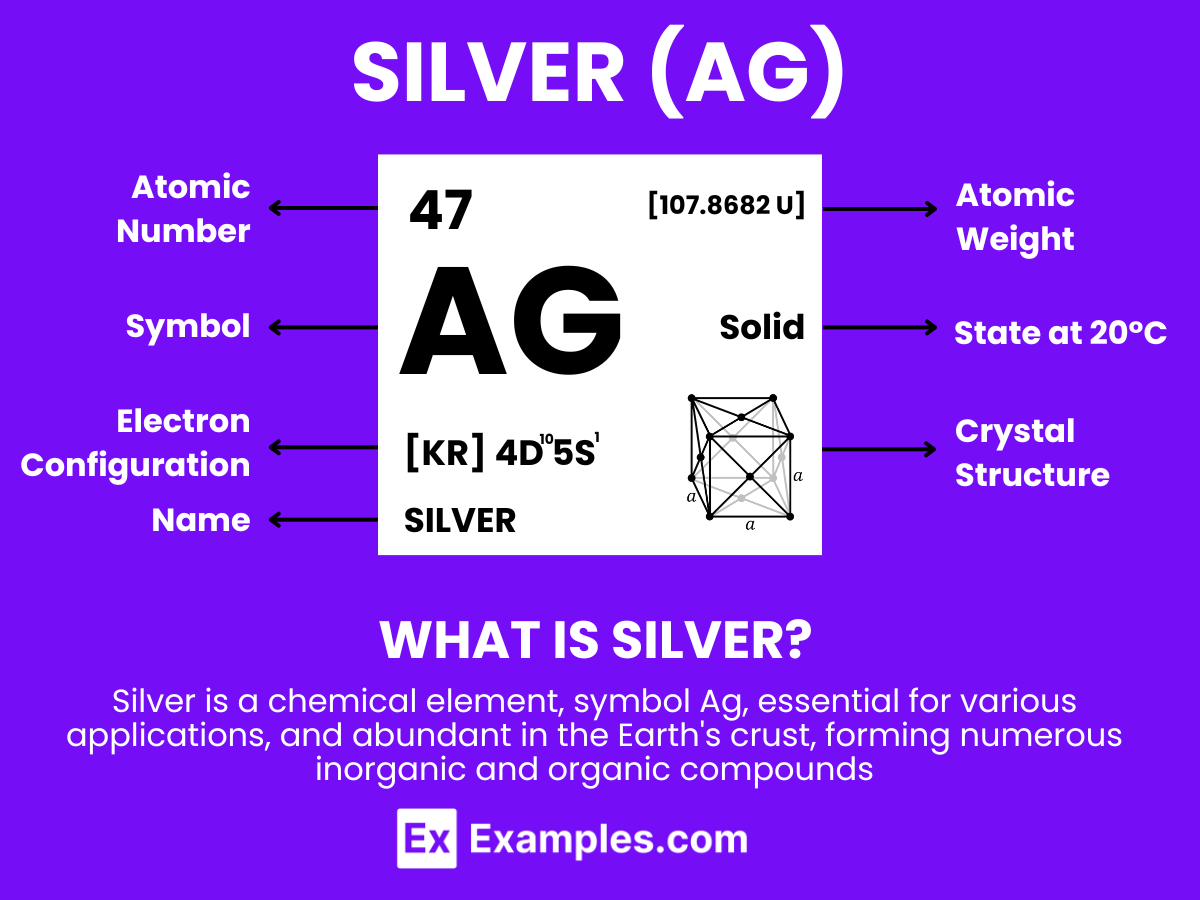
Silver is a chemical element with the symbol Ag and atomic number 47. It is a lustrous, white, soft, very ductile, and malleable metal. Known for its highest electrical and thermal conductivity of any metal, Silver is extensively used in a wide array of applications. Its inherent properties, such as reflectivity and resistance to corrosion, make it highly valuable not only for industrial purposes but also in jewelry, silverware, and photography. In industries like electronics, medicine, and energy, Silver plays a pivotal role due to its conductive and antimicrobial characteristics. .
Silver, symbol Ag (from the Latin ‘argentum’), with atomic number 47, is a precious metal known for its lustrious appearance and exceptional electrical conductivity. At an atomic level, silver exhibits a unique structure characterized by the following aspects:
The atomic structure of silver, with its filled d orbitals and single s orbital electron, plays a crucial role in its physical and chemical properties, making it invaluable in industries ranging from jewelry and coinage to electronics and photography.
| Property | Value |
|---|---|
| Appearance | Lustrous, white, metallic |
| Atomic Number | 47 |
| Atomic Weight | 107.8682 u |
| Density | 10.49 g/cm³ at 20 °C |
| Melting Point | 961.78 °C |
| Boiling Point | 2162 °C |
| Electrical Conductivity | Highest of all metals |
| Thermal Conductivity | 429 W/(m·K) at 25 °C |
| State at Room Temperature | Solid |
| Crystal Structure | Face-Centered Cubic (FCC) |
| Malleability | High, can be beaten into extremely thin sheets |
| Ductility | High, can be drawn into very thin wires |
| Reflectivity | Very high, reflects over 95% of the visible light spectrum |
Silver, with its symbol Ag, is a noble metal known for its low reactivity, but it still participates in several chemical reactions under specific conditions. Here are key chemical properties of silver:
| Property | Value |
|---|---|
| Melting Point | 961.78 °C |
| Boiling Point | 2162 °C |
| Heat of Fusion | 11.28 kJ/mol |
| Heat of Vaporization | 254 kJ/mol |
| Specific Heat Capacity | 25.35 J/(mol·K) at 25 °C |
| Property | Value |
|---|---|
| Density | 10.49 g/cm³ at 20 °C |
| Malleability | High, can be beaten into extremely thin sheets |
| Ductility | High, can be drawn into very thin wires |
| Tensile Strength | 170–200 MPa |
| Hardness (Mohs) | 2.5 – 3 |
| Thermal Expansion Coefficient | 18.9 µm/(m·K) at 25 °C |
| Property | Value |
|---|---|
| Electrical Conductivity | Highest of all metals, 63.01 x 10^6 S/m |
| Thermal Conductivity | 429 W/(m·K) at 25 °C |
| Magnetic Susceptibility | -19.5 x 10^-6 cm^3/mol (diamagnetic) |
| Property | Value |
|---|---|
| Natural Isotopes | Ag-107 (51.839%), Ag-109 (48.161%) |
| Radioisotopes | Ag-105, Ag-111, among others |
| Neutron Cross Section | 63.3 barns (for Ag-107) |
| Isotopic Abundance | Ag-107: 51.839%, Ag-109: 48.161% |
The preparation of silver involves extracting the metal from its ores and refining it to achieve high purity. Silver is commonly found in nature in its native form and as a part of various minerals such as argentite (silver sulfide) and horn silver (silver chloride).
| Isotope | Mass Number | Natural Abundance (%) | Half-life | Notes |
|---|---|---|---|---|
| Ag-107 | 107 | 51.839 | Stable | – |
| Ag-109 | 109 | 48.161 | Stable | – |
| Ag-105 | 105 | – | 41.2 days | Radioactive, used in research |
| Ag-111 | 111 | – | 7.45 days | Used in nuclear medicine |
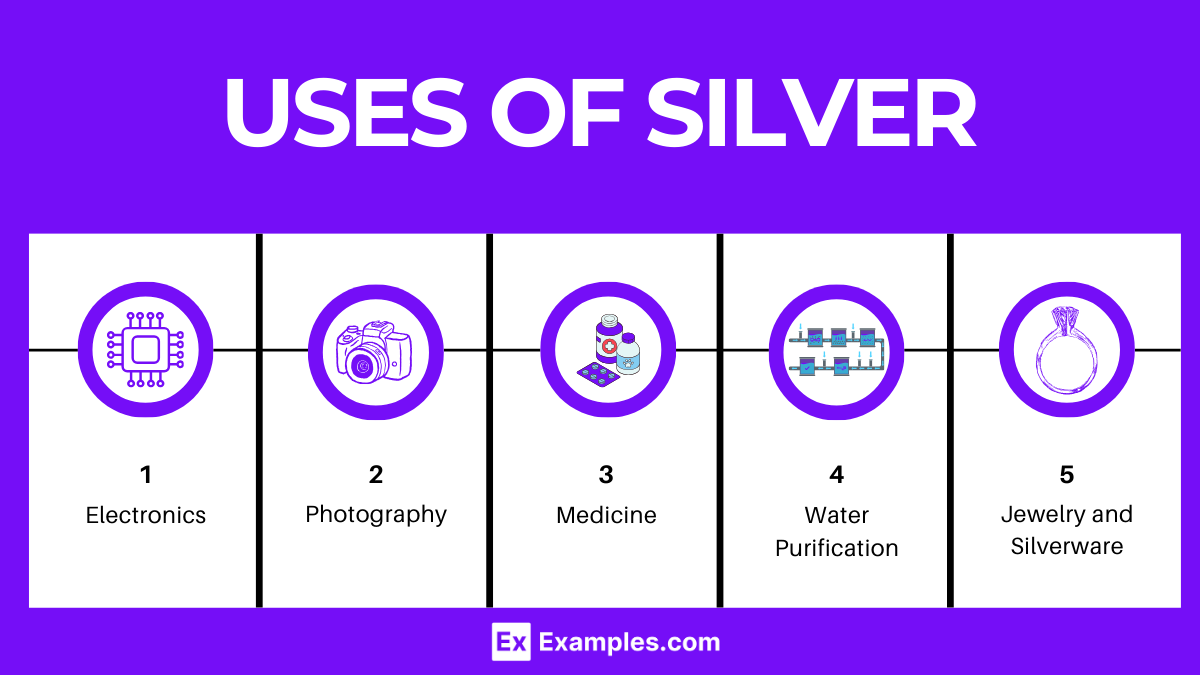
Silver’s unique properties, including its thermal and electrical conductivity, reflectivity, and antimicrobial nature, contribute to its wide-ranging applications:
Silver production involves several steps, from mining the ore to refining it into pure silver. The process can be summarized as follows:
Silver’s unique properties have made it indispensable in various fields:
silver, with its unmatched electrical conductivity, thermal properties, and antimicrobial qualities, remains a cornerstone in numerous industries. From electronics and medicine to jewelry and sustainable energy solutions, silver’s versatility and value are unparalleled. This table of silver not only highlights its importance but also underlines its indispensability in advancing modern technology and improving our daily lives.

Dive into the lustrous world of Silver, a precious metal that captivates with its versatility and brilliance. This comprehensive guide illuminates Silver’s rich history, its pivotal role in various industries, and the myriad of uses it offers, from jewelry making to technological applications. We’ll explore the elemental properties that make Silver so valuable, delve into its fascinating compounds, and showcase real-world examples. Whether for investment, industry, or innovation, Silver’s allure remains unmatched, promising endless possibilities.
Silver is a chemical element with the symbol Ag and atomic number 47. It is a lustrous, white, soft, very ductile, and malleable metal. Known for its highest electrical and thermal conductivity of any metal, Silver is extensively used in a wide array of applications. Its inherent properties, such as reflectivity and resistance to corrosion, make it highly valuable not only for industrial purposes but also in jewelry, silverware, and photography. In industries like electronics, medicine, and energy, Silver plays a pivotal role due to its conductive and antimicrobial characteristics. .
Formula: Ag
Composition: Consists of a single silver atom.
Bond Type: In its elemental form, silver does not have bonds as it is a pure element.
However, silver can form covalent or ionic bonds when reacting with other elements.
Molecular Structure: As a pure element, silver does not form a molecular structure in the same sense as compounds.
At room temperature, silver is in a metallic state with a face-centered cubic crystalline structure.
Electron Sharing: In compounds, silver typically forms ionic bonds by transferring electrons to other elements, though it can also participate in covalent bonding.
Significance: Silver is renowned for its high electrical and thermal conductivity, making it invaluable in electronics and solar panels. Its antimicrobial properties also make it essential in medical applications and water purification.
Role in Chemistry: Silver plays a crucial role in photography, electronics, and jewelry, among other applications. It forms various compounds, such as silver nitrate and silver sulfide, which are used in photographic film, conductive pastes, and antimicrobial agents, highlighting its versatility and importance in numerous fields.
Silver, symbol Ag (from the Latin ‘argentum’), with atomic number 47, is a precious metal known for its lustrious appearance and exceptional electrical conductivity. At an atomic level, silver exhibits a unique structure characterized by the following aspects:
Electron Configuration: The electron configuration of silver is [Kr] 4d¹⁰ 5s¹. This arrangement indicates that silver has a full d orbital and a single electron in the s orbital of its outermost shell, contributing to its high conductivity and malleability.
Atomic Radius: Silver atoms have an atomic radius of approximately 144 pm (picometers), which reflects the size of its electron cloud and influences its bonding characteristics.
Ionization Energy: The energy required to remove the most loosely held electron, the first ionization energy of silver, is about 731 kJ/mol, indicative of how easily silver can enter into chemical reactions.
Electronegativity: With a Pauling scale value of 1.93, silver exhibits moderate electronegativity, suggesting a balanced ability to attract electrons in chemical bonds.
Crystal Structure: At room temperature, silver crystallizes in a face-centered cubic (FCC) lattice structure, contributing to its high ductility and thermal conductivity. In this structure, each silver atom is surrounded by 12 others, providing the metal with its characteristic malleability and luster.
The atomic structure of silver, with its filled d orbitals and single s orbital electron, plays a crucial role in its physical and chemical properties, making it invaluable in industries ranging from jewelry and coinage to electronics and photography.
Property | Value |
|---|---|
Appearance | Lustrous, white, metallic |
Atomic Number | 47 |
Atomic Weight | 107.8682 u |
Density | 10.49 g/cm³ at 20 °C |
Melting Point | 961.78 °C |
Boiling Point | 2162 °C |
Electrical Conductivity | Highest of all metals |
Thermal Conductivity | 429 W/(m·K) at 25 °C |
State at Room Temperature | Solid |
Crystal Structure | Face-Centered Cubic (FCC) |
Malleability | High, can be beaten into extremely thin sheets |
Ductility | High, can be drawn into very thin wires |
Reflectivity | Very high, reflects over 95% of the visible light spectrum |
Silver, with its symbol Ag, is a noble metal known for its low reactivity, but it still participates in several chemical reactions under specific conditions. Here are key chemical properties of silver:
Tarnishing:Silver reacts with sulfur compounds in the air, leading to tarnishing.
Equation: 2Ag+H₂S+O₂→Ag₂S+H₂O
This forms a black layer of silver sulfide on the surface.
Reaction with Oxygen:Pure silver does not react with oxygen at room temperature, maintaining its lustrous appearance.
However, at high temperatures, silver can react with oxygen to form silver oxide.
Equation: 4Ag+O₂→2Ag₂O (at high temperatures)
Reaction with Acids:Silver is generally unreactive to hydrochloric acid and sulfuric acid under normal conditions.
It does, however, react with nitric acid to form silver nitrate.
Equation: 3Ag+4HNO₃→3AgNO₃+NO+2H₂O
Reaction with Halogens:Silver reacts with halogens to form silver halides, which are important in photographic processes.
Equation with Chlorine: 2Ag+Cl₂→2AgCl
Catalytic Properties:
Silver acts as a catalyst in certain chemical reactions, including the oxidation of alcohols.
It facilitates the breakdown of ethylene to ethylene oxide, a precursor for various organic compounds.
Formation of Complexes:
Silver forms complex ions with ammonia, cyanide, and other ligands.
Equation with Ammonia: Ag⁺+2NH₃→[Ag(NH₃)₂]⁺
These complexes are used in silver plating and as intermediates in the synthesis of other silver compounds.
Property | Value |
|---|---|
Melting Point | 961.78 °C |
Boiling Point | 2162 °C |
Heat of Fusion | 11.28 kJ/mol |
Heat of Vaporization | 254 kJ/mol |
Specific Heat Capacity | 25.35 J/(mol·K) at 25 °C |
Property | Value |
|---|---|
Density | 10.49 g/cm³ at 20 °C |
Malleability | High, can be beaten into extremely thin sheets |
Ductility | High, can be drawn into very thin wires |
Tensile Strength | 170–200 MPa |
Hardness (Mohs) | 2.5 – 3 |
Thermal Expansion Coefficient | 18.9 µm/(m·K) at 25 °C |
Property | Value |
|---|---|
Electrical Conductivity | Highest of all metals, 63.01 x 10^6 S/m |
Thermal Conductivity | 429 W/(m·K) at 25 °C |
Magnetic Susceptibility | -19.5 x 10^-6 cm^3/mol (diamagnetic) |
Property | Value |
|---|---|
Natural Isotopes | Ag-107 (51.839%), Ag-109 (48.161%) |
Radioisotopes | Ag-105, Ag-111, among others |
Neutron Cross Section | 63.3 barns (for Ag-107) |
Isotopic Abundance | Ag-107: 51.839%, Ag-109: 48.161% |
The preparation of silver involves extracting the metal from its ores and refining it to achieve high purity. Silver is commonly found in nature in its native form and as a part of various minerals such as argentite (silver sulfide) and horn silver (silver chloride).
Cyanide Process:
Silver is extracted from its ores using a cyanide solution. Silver ore is treated with a dilute cyanide solution, which dissolves the silver.
Equation:Ag₂S+4NaCN→2Na[Ag(CN)₂]+Na₂S
Silver is then recovered from the solution by adding zinc.
Equation:2Na[Ag(CN)₂]+Zn→Na₂[Zn(CN)₄]+2Ag
Amalgamation Process:
This method involves mixing silver-containing ores with mercury, where silver forms an amalgam with mercury.
The amalgam is then heated, evaporating the mercury, leaving behind pure silver.
Ag₂S+2Hg→(AgHg)₂+S
Electrolytic Refining:
Silver is refined electrolytically by dissolving impure silver in an electrolyte solution and using it as the anode. A pure silver plate is used as the cathode.
Silver ions move towards the cathode and deposit as pure silver.
Equation: At anode: Ag→Ag⁺+e⁻
Equation: At cathode: Ag⁺+e⁻→Ag
Parkes Process:
Used for removing silver from lead during the production of bullion. Zinc is added to molten lead containing silver, where silver preferentially binds to zinc.
The zinc-silver alloy is then distilled to separate the silver.
Equation:Zn+Ag(inlead)→(Ag−Zn)
Silver Nitrate (AgNO₃)
A versatile compound used in photography, medicine, and as a reagent in laboratory synthesis. It forms when silver reacts with nitric acid.
Equation: 3Ag+4HNO₃→3AgNO₃+NO+2H₂O
Silver Chloride (AgCl)
Appears as a white crystalline solid, pivotal in photographic films and as a precursor to other silver compounds. It’s produced by reacting silver nitrate with a chloride source.
Equation:3AgNO₃+NaCl→AgCl+NaNO₃
Silver Oxide (Ag₂O)
Used in batteries and as a mild oxidizing agent in organic synthesis. It forms from the oxidation of silver or by reacting silver nitrate with a base.
Equation: 2AgNO₃+2NaOH→Ag₂O+2NaNO₃+H₂O
Silver Sulfide (Ag₂S)
Known for causing tarnish on silver surfaces, this compound results from silver’s reaction with sulfur or hydrogen sulfide.
Equation: 2Ag+H₂S→Ag₂S+H₂
Silver Iodide (AgI)
Utilized in cloud seeding to induce rain and in photographic emulsions. It forms by reacting silver nitrate with potassium iodide.
Equation: 3AgNO₃+KI→AgI+KNO₃
Silver Cyanide (AgCN)
Used in silver plating and in the synthesis of other silver-based chemicals. It forms when silver nitrate reacts with potassium cyanide.
Equation: AgNO₃+KCN→AgCN+KNO₃
Isotope | Mass Number | Natural Abundance (%) | Half-life | Notes |
|---|---|---|---|---|
Ag-107 | 107 | 51.839 | Stable | – |
Ag-109 | 109 | 48.161 | Stable | – |
Ag-105 | 105 | – | 41.2 days | Radioactive, used in research |
Ag-111 | 111 | – | 7.45 days | Used in nuclear medicine |

Silver’s unique properties, including its thermal and electrical conductivity, reflectivity, and antimicrobial nature, contribute to its wide-ranging applications:
Electronics: Due to its excellent electrical conductivity, silver is used in electrical contacts and conductors.
Jewelry and Silverware: Its lustrous appearance and malleability make silver a popular choice for jewelry, cutlery, and decorative items.
Photography: Silver halides are sensitive to light, making silver compounds essential in traditional photographic film and paper.
Medicine: Silver’s antimicrobial properties make it valuable in wound dressings, medical devices, and as a coating to prevent bacterial growth.
Water Purification: Silver ions are used in water filters to disinfect water by killing bacteria and other pathogens.
Catalysis: Silver catalyzes many chemical reactions, including the production of formaldehyde and the oxidation of ethylene to ethylene oxide.
Currency: Historically, silver has been used to mint coins and remains a standard for monetary systems in many countries.
Silver production involves several steps, from mining the ore to refining it into pure silver. The process can be summarized as follows:
Mining: Silver is extracted from the earth primarily through mining. It is found both in its free form and as a part of various minerals. The primary sources of silver include silver ores, lead, lead-zinc, copper, copper-nickel, gold, and byproducts of metallurgy processes.
Ore Processing: The extracted ore is crushed and ground to liberate the silver-containing minerals. It is then subjected to froth flotation to concentrate the silver ore.
Smelting and Refining: The concentrated ore is then smelted to remove impurities, producing lead bullion bars that contain silver. The Parkes process is often used to extract silver from lead during this stage. Finally, the silver is refined through electrolysis or the cupellation process to achieve purity levels suitable for commercial use.
Recycling: A significant amount of silver comes from recycling, including photographic materials, jewelry, and industrial byproducts, contributing to the overall supply.
Silver’s unique properties have made it indispensable in various fields:
Electronics: Due to its unparalleled electrical conductivity, silver is widely used in electrical contacts and conductors, including switches, solar panels, and batteries.
Jewelry and Silverware: Silver’s luster and ductility make it a popular choice for jewelry, decorative items, and tableware.
Photography: Although digital photography has reduced the demand for silver in photographic film, it remains a component in x-ray films and specialized photography.
Medicine: Silver’s antimicrobial properties are utilized in medical devices, wound dressings, and coatings to prevent bacterial infections.
Industrial Applications: Silver is used in brazing and soldering, as well as in mirror production due to its high reflectivity.
Coinage and Investment: Silver coins and bullion are popular forms of investment and currency due to their intrinsic value.
silver, with its unmatched electrical conductivity, thermal properties, and antimicrobial qualities, remains a cornerstone in numerous industries. From electronics and medicine to jewelry and sustainable energy solutions, silver’s versatility and value are unparalleled. This table of silver not only highlights its importance but also underlines its indispensability in advancing modern technology and improving our daily lives.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Silver?
46
47
48
49
What is the chemical symbol for Silver?
Ag
Au
Si
Sb
Silver is primarily extracted from which ore?
Hematite
Bauxite
Galena
Argentite
Which property of Silver makes it ideal for use in electrical contacts?
High melting point
High thermal conductivity
High electrical conductivity
High density
What is the molar mass of Silver?
107.87 g/mol
108.87 g/mol
109.87 g/mol
110.87 g/mol
Silver nitrate is commonly used in which field?
Agriculture
Photography
Medicine
Construction
Which alloy contains Silver and is used in jewelry?
Brass
Bronze
Sterling silver
Pewter
What is the primary use of Silver in the medical field?
Surgical instruments
Antimicrobial agents
Diagnostic equipment
Implants
Which property of Silver makes it useful in mirrors?
High reflectivity
Low density
High hardness
Low melting point
What is the melting point of Silver?
800.0°C
1200.0°C
500.0°C
961.8°C
Before you leave, take our quick quiz to enhance your learning!

