What is the molar mass of sodium chloride?
58.44 g/mol
45.89 g/mol
45.89 g/mol
100.22 g/mol

Sodium Chloride (NaCl), commonly known as table salt, is a prime example of ionic compounds, playing a pivotal role in both our daily lives and the broader spectrum of chemistry education. As teachers guiding the next generation of scientific minds, understanding the intricate balance of sodium and chlorine ions in forming this essential compound offers a tangible way to illustrate the principles of ionic bonding and chemical stability. This guide aims to equip educators with comprehensive insights and practical examples to demystify the concept of ionic compounds through the lens of sodium chloride, enhancing their teaching toolkit for a more engaging and informative classroom experience.
| Formula | NaCl |
| Hill Formula | ClNa |
| Name | Sodium Chloride |
| Alternate Names | Common Salt, Rock Salt, Salt, Table Salt |

The structure of Sodium Chloride (NaCl) is characterized by a crystalline lattice composed of sodium (Na⁺) and chloride (Cl⁻) ions, arranged in a cubic pattern known as a face-centered cubic (FCC) lattice. In this arrangement, each sodium ion is surrounded by six chloride ions, and similarly, each chloride ion is surrounded by six sodium ions. This symmetrical, three-dimensional arrangement results in a highly stable and tightly packed structure.
The ions in Sodium Chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride ions. This type of bonding contributes to the high melting and boiling points of NaCl, as well as its solubility in water. When NaCl dissolves in water, the ionic bonds are broken as the individual sodium and chloride ions are surrounded by water molecules, allowing them to disperse evenly throughout the solution.
This crystalline structure of Sodium Chloride is not only fundamental to its chemical properties but also to its wide range of applications, from culinary uses to industrial and scientific applications. The understanding of NaCl’s structure is crucial for students and educators in chemistry, providing a classic example of ionic bonding and crystal lattice structure.
The chemical formula of Sodium Chloride is NaCl. This formula indicates that each unit of Sodium Chloride is composed of one sodium ion (Na⁺) and one chloride ion (Cl⁻), representing the simplest ratio of these two ions in the compound.
Sodium Chloride (NaCl), commonly known as salt, is one of the most abundant minerals on Earth, primarily found in seawater and salt mines. In seawater, NaCl is dissolved, contributing to the ocean’s salinity, which averages about 3.5%. This vast resource is harvested through evaporation ponds, where seawater is allowed to evaporate, leaving behind crystallized salt.
Beyond the oceans, large deposits of solid rock salt can be found underground in salt mines. These deposits were formed millions of years ago from the evaporation of ancient bodies of water. Today, these mines provide a significant source of Sodium Chloride for industrial, culinary, and other uses. Countries with significant salt mines include the United States, China, Germany, and Canada.
Sodium Chloride is also present in small quantities in many minerals and natural springs, contributing to the mineral content of various waters. Its widespread occurrence and ease of extraction make it readily available for a multitude of applications, from food seasoning and preservation to chemical production and de-icing roads.
Sodium chloride (NaCl) is commonly prepared through the combination of sodium hydroxide (NaOH) with hydrochloric acid (HCl) in a neutralization reaction. The balanced chemical equation for this process is:
Here, sodium hydroxide reacts with hydrochloric acid to produce sodium chloride and water. Another method involves the reaction between sodium metal and chlorine gas, yielding sodium chloride. This reaction is represented as: 2Na(s) + Cl₂(g) → 2NaCl(s). Both methods result in the formation of sodium chloride, a vital compound used in various industrial processes and as a common table salt in everyday life.
| Property | Description |
|---|---|
| Appearance | White crystalline solid |
| Odor | Odorless |
| Taste | Salty |
| Molecular Weight | 58.44 g/mol |
| Density | 2.16 g/cm³ (solid) |
| Melting Point | 801°C (1474°F) |
| Boiling Point | 1413°C (2575°F) |
| Solubility in Water | 35.9 g/100 mL (0°C); 36.0 g/100 mL (20°C); 39.12 g/100 mL (100°C) |
| Solubility | Soluble in water, glycerol; slightly soluble in ethanol; practically insoluble in acetone |
| Refractive Index | 1.5442 (Solid) |
| Crystal Structure | Face-centered cubic (FCC) |
| Thermal Conductivity | 6.5 W/(m·K) (Solid) |
NaCl is extremely stable due to the strong ionic bond between the sodium (Na+) and chloride (Cl−) ions. This stability makes it non-reactive under normal conditions.
NaCl reacts with sulfuric acid (H₂SO₄) to produce hydrochloric acid (HCl) and sodium bisulfate (NaHSO₄) at lower concentrations. At higher concentrations and temperatures, this reaction can lead to the formation of sodium sulfate (Na₂SO₄). NaCl (s) + H₂SO4 (l) → NaHSO4 (s) + HCl (g)NaCl (s) + H₂SO4 (l) → NaHSO₄ (s) + HCl (g)
When reacted with a strong base like sodium hydroxide (NaOH), especially at high temperatures, it can form sodium hypochlorite (NaClO), sodium chlorate (NaClO₃), or even sodium perchlorate (NaClO₄) depending on the reaction conditions. 2NaOH (aq) + Cl₂(g) → NaCl (aq) + NaClO (aq) + H₂O (l)2NaOH (aq) + Cl₂(g) → NaCl (aq) + NaClO (aq) + H₂O (l)
NaCl is highly soluble in water, and its solubility varies with temperature. Upon dissolving, it dissociates into its constituent ions (Na+ and Cl−), which are essential for conducting electricity, making its aqueous solution an excellent electrolyte. NaCl (s) → Na+(aq) + Cl−(aq)NaCl (s) → Na+(aq) + Cl⁻ (aq)
One of the hallmark chemical tests for chloride ions is the reaction with silver nitrate, producing a white precipitate of silver chloride (AgCl). NaCl (aq) + AgNO₃(aq) → AgCl (s) + NaNO₃ (aq)NaCl (aq) + AgNO₃ (aq) → AgCl (s) + NaNO₃ (aq) This reaction is utilized in analytical chemistry to detect the presence of chloride ions.
Although NaCl is stable at high temperatures, it can be decomposed into sodium metal and chlorine gas at very high temperatures, well above its melting point, in an electrolysis process. 2NaCl (l) → 2Na (l) + Cl₂(g)2NaCl (l) → 2Na (l) + Cl₂(g) This process is primarily used in the industrial production of chlorine gas and sodium metal.
| Thermodynamic Property | Value (Units) |
|---|---|
| Specific Heat Capacity | 0.864 J/g°C |
| Molar Heat Capacity | 51.21 J/mol°C |
| Specific Gibbs Energy of Formation | -385.92 kJ/mol |
| Specific Enthalpy of Formation | -411.12 kJ/mol |
| Molar Enthalpy of Formation | -385.92 kJ/mol |
| Specific Entropy | 51.98 J/mol°C |
| Molar Entropy | 88.70 J/mol°C |
| Specific Heat of Fusion | 3.86 kJ/mol |
| Molar Heat of Fusion | 28.5 kJ/mol |
| Identifier | Number/Code |
|---|---|
| CAS registry number | 7647-14-5 |
| Beilstein number | 3534976 |
| PubChem compound ID | 5234 |
| PubChem substance ID | 24852266 |
| SMILES identifier | [Na+].[Cl-] |
| InChI identifier | InChI=1/ClH.Na/h1H;/q;+1/p-1/fCl.Na/h1h;/q-1;m |
| RTECS number | VZ4725000 |
| MDL number | MFCD00003477 |
| NFPA Rating | Value |
|---|---|
| NFPA health rating | 1 |
| NFPA fire rating | 0 |
| NFPA reactivity rating | 0 |
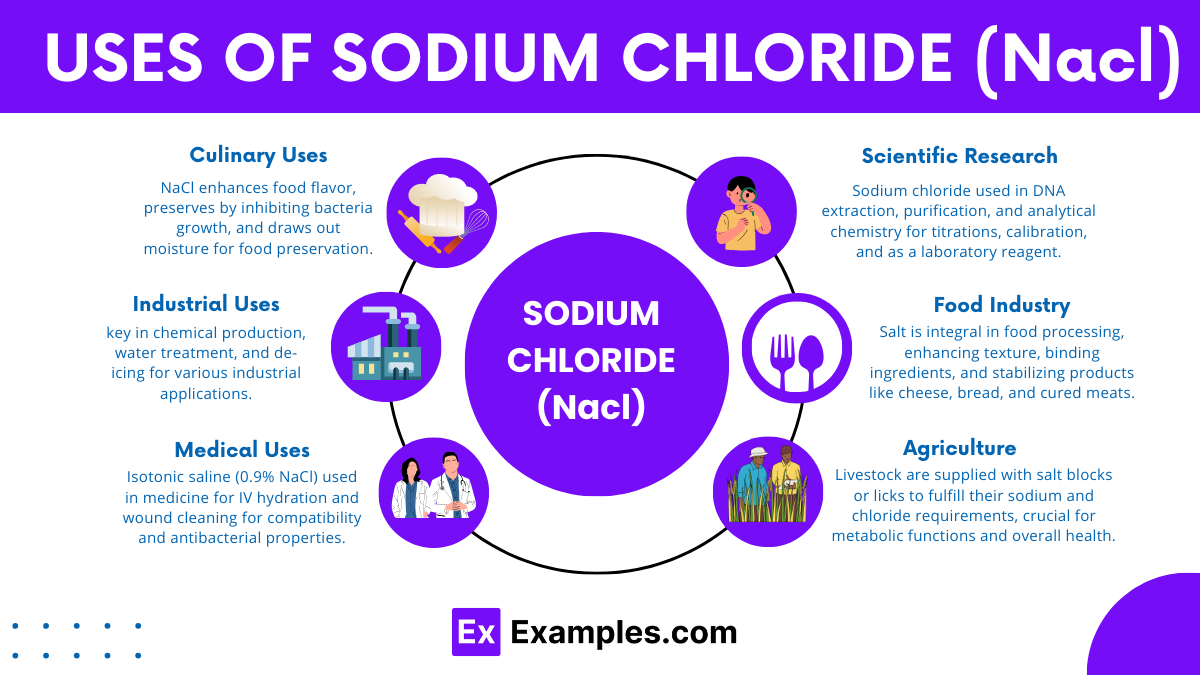
Sodium Chloride (NaCl), commonly known as salt, plays a crucial role in a wide range of applications spanning culinary, industrial, medical, and scientific fields. Its versatility and abundance make it a staple compound across various sectors. Here’s a detailed exploration of its multifaceted uses:
Sodium Chloride (NaCl), or table salt, is essential for various physiological processes in the human body, playing a critical role in maintaining health and well-being. Here’s a detailed overview of its key functions:
Sodium Chloride is crucial in maintaining the osmotic balance in the body. It helps regulate the body’s fluid balance, the regulation of body fluid balance involves understanding the tonicity of body fluids, the balance and abnormalities of sodium salt and extracellular volume, and the challenges of assessing extracellular volume in severe illnesses, highlighting the need for further research in this area.
NaCl plays a pivotal role in transmitting nerve signals. Sodium ions (Na+) are essential for the conduction of electrical signals in the nervous system. These signals are crucial for muscle contractions, reflexes, and various neural functions.
Similar to nerve function, sodium ions are vital for muscle contraction. Table salt, a key ingredient in Thanksgiving dishes, is a compound of sodium and chloride, while sodium is also used in various forms, including baking soda, and in the production of soaps, cosmetics, paper, glass, metals, and medicines.
Sodium Chloride aids in the absorption and transport of certain nutrients in the small intestine. Sodium-dependent transporters help
absorb glucose and amino acids, which are crucial for energy and protein synthesis.
Sodium plays a key role in determining blood volume and pressure. While excessive sodium intake can lead to high blood pressure, an appropriate amount is necessary for maintaining blood volume and, by extension, blood pressure within normal ranges.
Sodium Chloride contributes to the body’s acid-base balance. It is part of the buffer systems that help maintain the blood and other bodily fluids’ pH within the narrow range necessary for proper cellular function.
| Feature | Salt (Sodium Chloride, NaCl) | Sodium (Na) |
|---|---|---|
| Composition | Salt is a compound consisting of sodium (Na+) ions and chloride (Cl−) ions. | Sodium is a chemical element, represented by the symbol Na and atomic number 11. |
| Physical State | At room temperature, salt is a solid, crystalline substance. | Sodium is a soft, silvery-white, highly reactive metal. |
| Usage | Used mainly as a seasoning in food, as well as in industrial applications and water treatment. | Used in various industrial processes, including the production of chemicals, in metallic form, and in sodium vapor lamps. |
| Reactivity | Salt is stable and non-reactive under normal conditions due to the ionic bond between sodium and chloride ions. | Sodium is highly reactive, especially with water, where it reacts vigorously producing hydrogen gas and heat. |
| Health Impact | Essential for human health in moderate amounts, regulating fluid balance and blood pressure. Excessive consumption can lead to health issues. | Sodium is essential for human health, particularly in nerve impulse transmission and muscle function, but it is not used in its metallic form due to its reactivity. |
| Occurrence | Found naturally in seawater and underground deposits. It can also be manufactured through the evaporation of seawater. | Sodium is abundant in the Earth’s crust and is found in many minerals, but not in its free, metallic state due to its reactivity. |
| Handling | Safe to handle and consume in its common form as table salt. | Requires careful handling due to its reactivity, particularly with moisture and air. |
Sodium chloride and saline are not the same; saline is a solution of sodium chloride (salt) and water, commonly used for medical purposes.
Consuming sodium chloride, or table salt, in moderate amounts is essential for fluid balance and nerve function, but excessive intake can lead to health issues like hypertension.
In skincare, sodium chloride serves as a thickener or to maintain the product’s isotonicity; however, it can be drying for some skin types.
Sodium chloride solutions (saline) are given to individuals to replenish lost fluids, correct electrolyte imbalances, administer drugs intravenously, or cleanse wounds.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the molar mass of sodium chloride?
58.44 g/mol
45.89 g/mol
45.89 g/mol
100.22 g/mol
What is the crystal structure of sodium chloride?
Face-centered cubic
Body-centered cubic
Hexagonal close-packed
Simple cubic
What is the solubility of sodium chloride in water at room temperature?
35.7 g/L
357 g/L
0.357 g/L
3.57 g/L
What is the melting point of sodium chloride?
801°C
100°C
500°C
900°C
Which ions are present in an aqueous solution of sodium chloride?
Na2+ and Cl2-
Na+ and Cl-
Na- and Cl+
Na and Cl
Sodium chloride is also known as:
Baking soda
Epsom salt
Table salt
Epsom salt
What is the boiling point of sodium chloride?
100°C
1413°C
800°C
1625°C
Which process is used to obtain pure sodium chloride from seawater?
Distillation
Filtration
Evaporation
Chromatography
Sodium chloride is essential for human health because it helps to:
Build bones
Carry oxygen in the blood
Regulate fluid balance
Produce hormones
What type of reaction occurs when sodium reacts with chlorine to form sodium chloride?
Decomposition
Combination
Single displacement
Double displacement
Before you leave, take our quick quiz to enhance your learning!

