What is the chemical formula of Sodium Hydroxide?
NaOH
NaCl
H2SO4
NH3

Sodium hydroxide, also known as lye or caustic soda, is a powerful base commonly used in various industries and everyday products. This white, solid compound is highly soluble in water, producing heat upon dissolution. It plays a crucial role in manufacturing paper, soap, and detergents, and is also used in water treatment processes to help neutralize acids and control water acidity. Despite its many uses, sodium hydroxide must be handled with care due to its ability to cause severe burns upon contact with skin, necessitating safety precautions during use. This compound’s versatility makes it a staple in both industrial applications and household cleaning tasks, highlighting its importance in science and industry.
| Property | Value |
|---|---|
| Formula | NaOH |
| Hill Formula | HNaO |
| Name | Sodium hydroxide |
| Alternate Names | Aetznatron, Ascarite, Caustic Soda, Collo-Grillrein, Collo-Tapetta, Lye, Soda Lye, Sodium Hydrate, White Caustic |

Sodium hydroxide, also known as NaOH, has a simple yet fundamental chemical structure in the world of chemistry. It is composed of sodium ions (Na⁺) and hydroxide ions (OH⁻) held together by ionic bonds. In its solid state, sodium hydroxide forms a crystalline structure, which means the ions are arranged in a specific and orderly pattern. This arrangement allows it to dissolve readily in water, where the sodium and hydroxide ions separate and move freely. This dissociation is crucial for its function as a base, enabling it to react with acids and other substances effectively. Its ionic nature and strong base properties make it essential for a wide range of industrial and laboratory applications.
Sodium hydroxide, commonly known as lye, can be produced using several methods, but one of the most common and efficient is through the electrolysis of salt water, a process known as the chloralkali process. This method involves passing an electric current through a solution of sodium chloride (common table salt dissolved in water). The chemical reaction that occurs breaks down the salt into its components.
The chemical equation for this process is:
In this reaction, chlorine gas (Cl₂) is released at one electrode (anode), and hydrogen gas (H₂) is released at the other electrode (cathode), while sodium hydroxide remains in solution. This method not only produces sodium hydroxide but also valuable chlorine and hydrogen gases, which are used in various industrial applications. This electrolytic approach is favored for its efficiency and ability to produce high-purity sodium hydroxide, crucial for its many uses in industries ranging from manufacturing to cleaning products.
| Property | Description |
|---|---|
| Appearance | White, odorless, crystalline solid |
| Solubility | Highly soluble in water, forms a heat-generating solution |
| Melting Point | 318°C (604°F) |
| Boiling Point | 1,388°C (2,530°F) |
| Density | 2.13 g/cm³ at 20°C (68°F) |
| pH | Very high, typically around 14 when in solution |
| Thermal Stability | Stable up to its melting point but decomposes upon heating above |
| Property | Description |
|---|---|
| CAS registry number | 1310-73-2 |
| PubChem compound ID | 14798 |
| SMILES identifier | [OH-].[Na+] |
| InChI identifier | InChI=1/Na.H2O/h;1H2/q+1;/p-1/fNa.HO/h;1h/qm;-1 |
| MDL number | MFCD00132264 |
| Property | Rating |
|---|---|
| NFPA health rating | 4 |
| NFPA fire rating | 0 |
| NFPA reactivity rating | 4 |
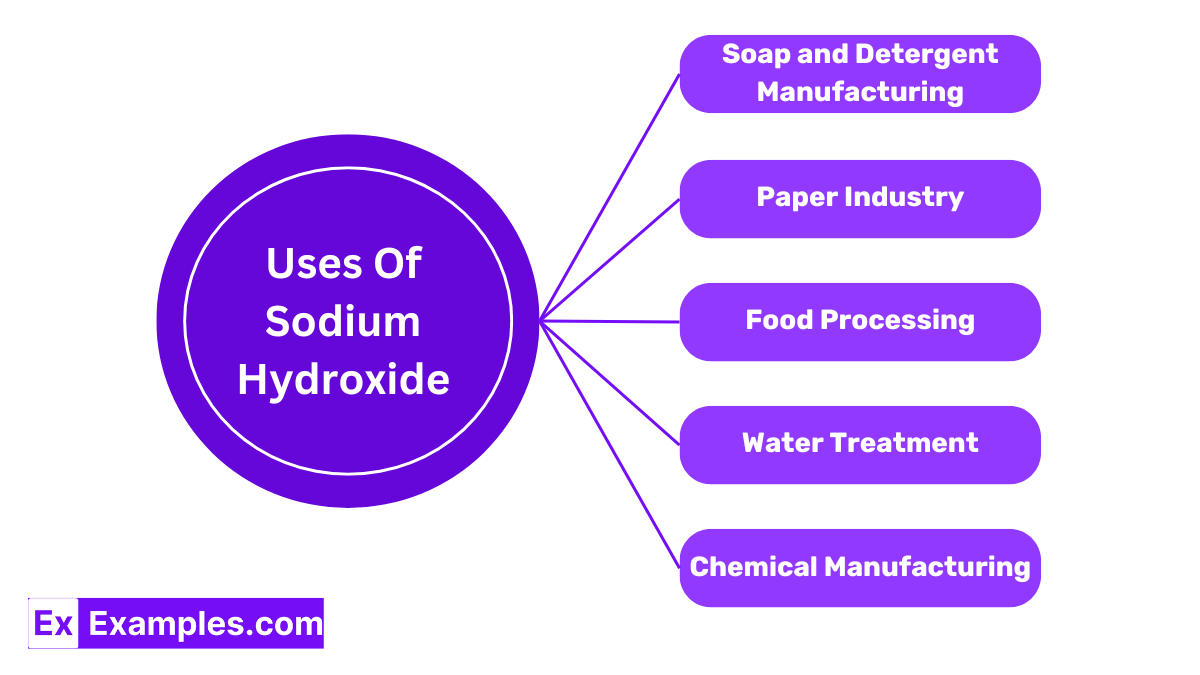
Sodium hydroxide is critical in the production of soaps and detergents. It reacts with fats and oils in a process called saponification to create soap. This reaction transforms triglycerides into glycerol and fatty acid salts, which are the cleansing agents in soaps and detergents.
In the papermaking process, sodium hydroxide is used to separate cellulose fibers from lignin, the ‘glue’ that holds the wood fibers together. This chemical is essential for breaking down wood into pulp, leading to the production of smoother, higher-quality paper.
Sodium hydroxide plays a crucial role in water treatment facilities. It is used to adjust the pH level of water, making it more alkaline to facilitate the removal of impurities and heavy metals through precipitation.
In the food industry, sodium hydroxide is used in the cleaning and sanitation process of equipment. It’s also employed in the pretreatment of certain foods, like olives, which are soaked in a lye solution to soften them and reduce bitterness.
As a reagent, sodium hydroxide is instrumental in various chemical reactions. It is used to produce an array of chemicals, including solvents, plastics, synthetic fibers, and bleach.
Yes, sodium hydroxide can be very harmful, causing severe skin burns, eye damage, and respiratory issues if mishandled.
No, sodium hydroxide (NaOH) and baking soda are different compounds with distinct chemical properties and uses.
The risks include severe burns, respiratory problems, eye injuries, and systemic effects if ingested or improperly handled.
No, sodium hydroxide is a strong alkaline compound, not just salt water, which typically refers to a solution of sodium chloride (table salt) in water.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of Sodium Hydroxide?
NaOH
NaCl
H2SO4
NH3
Sodium Hydroxide is commonly known as:
Baking soda
Lime
Lye
Bleach
Which of the following is a major use of Sodium Hydroxide?
Water purification
Making glass
Producing paper
Strengthening fabrics
Sodium Hydroxide is a:
Weak acid
Strong acid
Weak base
Strong base
What safety precaution is necessary when handling Sodium Hydroxide?
Use of a magnet
Wearing insulated gloves
Wearing protective eyewear
Use of a catalyst
Sodium Hydroxide reacts with fats to produce:
Alcohol
Soap
Glycerin
Acid
In which industry is Sodium Hydroxide NOT commonly used?
Textile
Petroleum
Agriculture
Cosmetics
The neutralization of Sodium Hydroxide requires:
An indicator
A buffer
An acid
A base
Exposure to Sodium Hydroxide can cause:
Drowsiness
Skin burns
Hair growth
Decreased heart rate
Sodium Hydroxide is solid at room temperature. Its appearance is most commonly:
Colorless crystals
Red liquid
Yellow pellets
Blue flakes
Before you leave, take our quick quiz to enhance your learning!

