What is the atomic number of Sodium?
10
11
12
13
Sodium, an essential alkali metal, plays a pivotal role in both the scientific world and our daily lives. This comprehensive guide delves into the fundamental aspects of sodium, exploring its properties, diverse applications, and critical safety tips. Perfect for teachers, it provides practical examples to enrich classroom discussions, from its role in the human body to its use in industries. Understanding sodium’s dynamics enhances educational experiences, making science both relatable and intriguing.
Sodium is a highly reactive, silvery-white alkali metal, known for its abundance in the Earth’s crust and oceans. It’s essential for various biological functions and is widely used in compounds like sodium chloride (table salt). In its pure form, sodium reacts swiftly with water and air, necessitating careful handling. Its properties and compounds make it a fundamental element in both chemistry education and various industrial applications.
| Lithium |
| Potassium |
| Rubidium |
| Cesium |
| Francium |
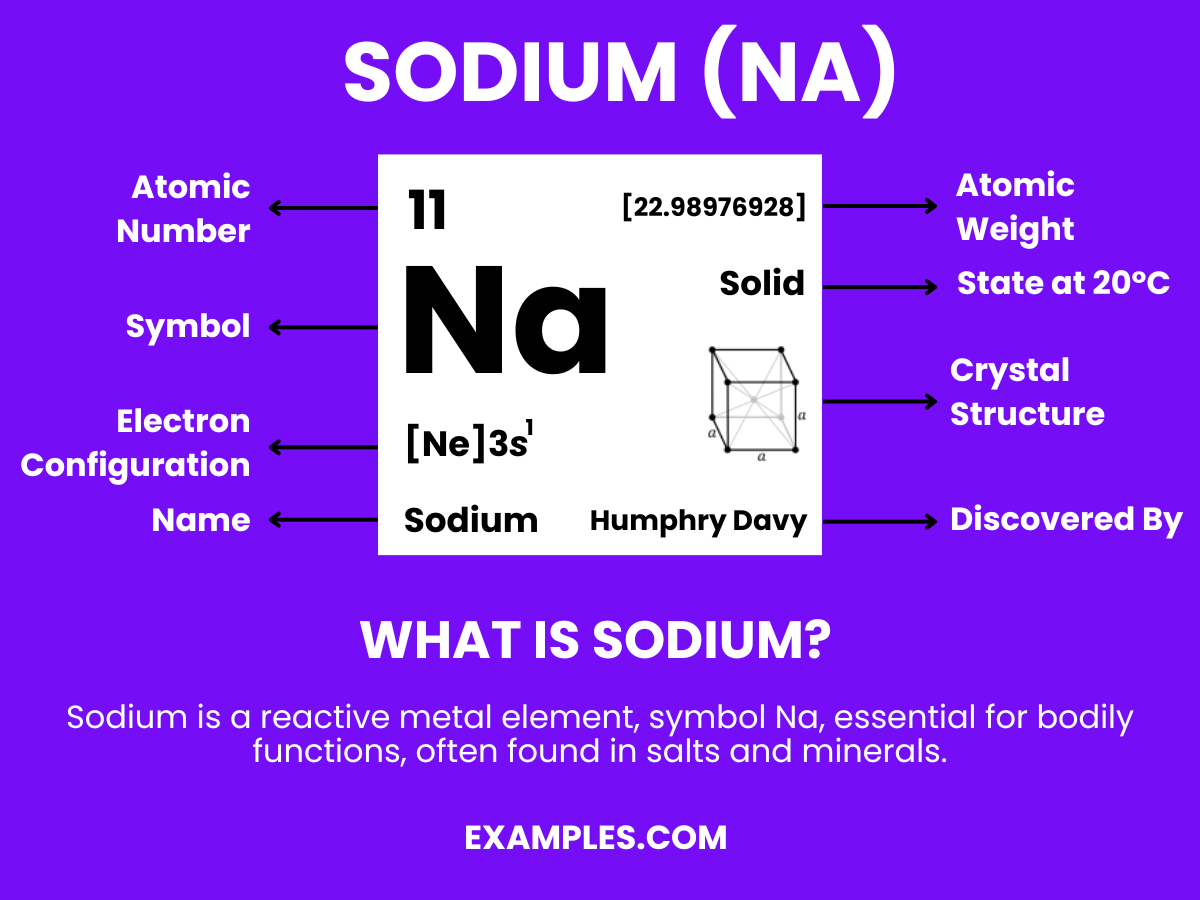
Formula: Na
Composition: A single sodium atom.
Bond Type: Highly reactive, especially with water.
Molecular Structure: Soft metal.
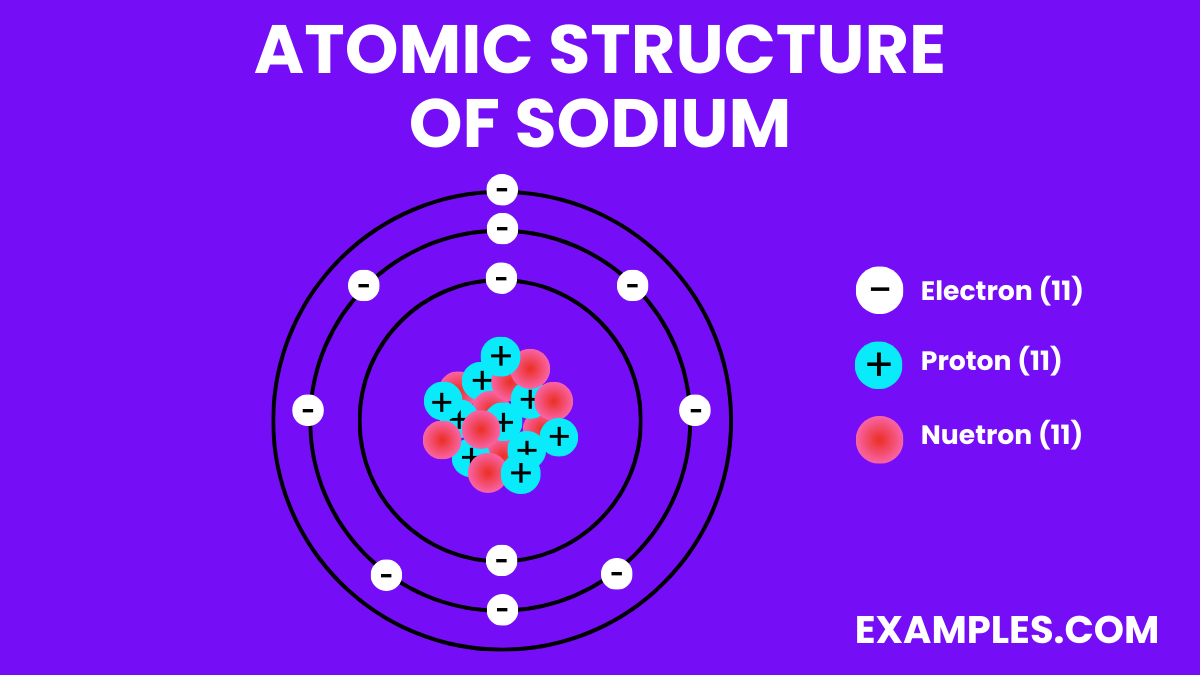
Electron Configuration: 11 electrons; configuration 1s² 2s² 2p⁶ 3s¹.
Significance: Essential in daily life, used in table salt and industrial applications.
Role in Chemistry: Forms vital compounds like sodium chloride (NaCl).
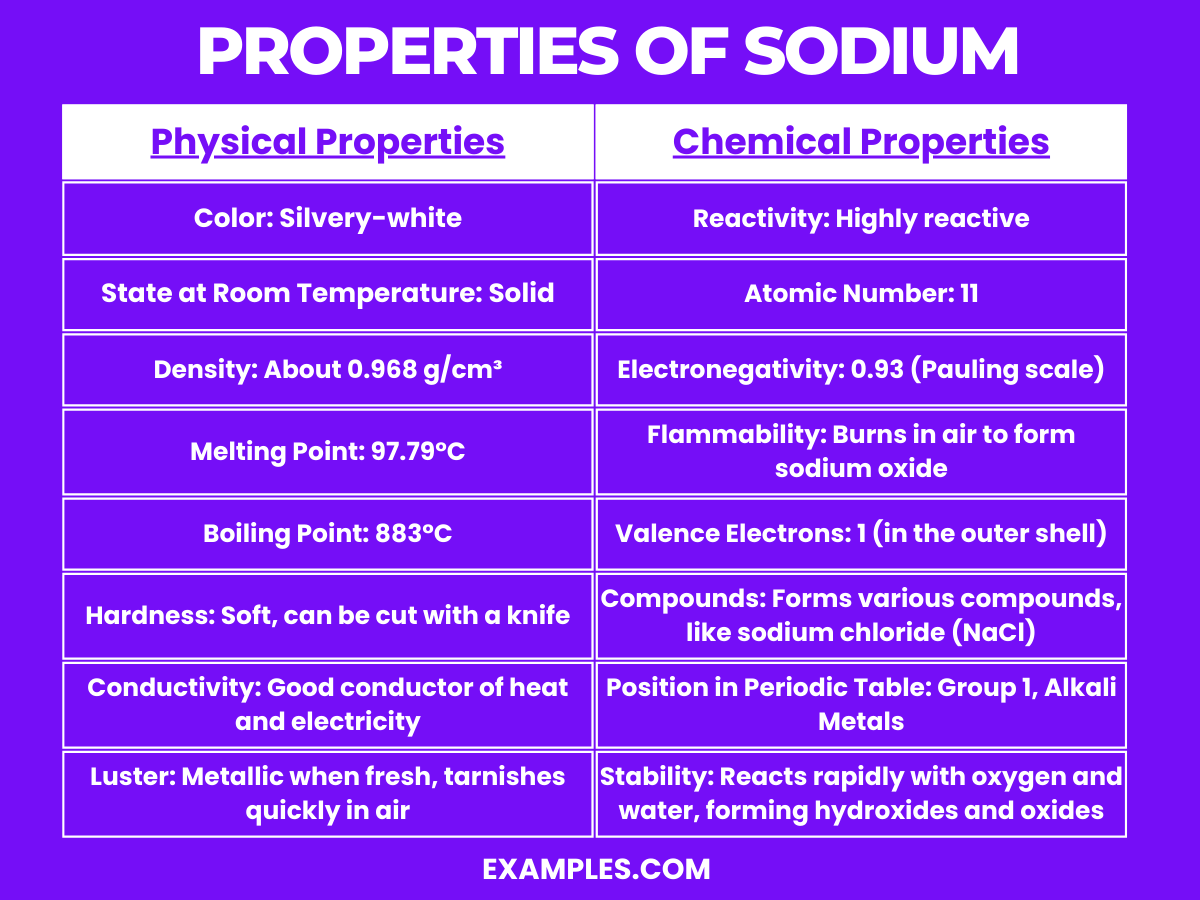
| Physical Property | Description |
|---|---|
| Color | Silvery-white |
| State at Room Temperature | Solid |
| Density | About 0.968 g/cm³ |
| Melting Point | 97.79°C (208°F) |
| Boiling Point | 883°C (1621°F) |
| Hardness | Soft, can be cut with a knife |
| Conductivity | Good conductor of heat and electricity |
| Luster | Metallic when fresh; tarnishes quickly in air due to oxidation |
Sodium exhibits several notable chemical properties:
| Property | Value with Unit |
|---|---|
| Boiling Point | 883 °C |
| Melting Point | 97.79 °C |
| Critical Temperature | 2573 °C |
| Critical Pressure | 35 MPa |
| Heat of Vaporization | 97.42 kJ/mol |
| Heat of Fusion | 2.60 kJ/mol |
| Specific Heat Capacity (at 25°C) | 1.23 J/g·K |
| Thermal Conductivity | 142 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 20°C) | 968 kg/m³ |
| Viscosity (at 100°C) | 0.71 mPa·s |
| Solubility in Water | Reacts with water |
| Refractive Index | NA (Metallic) |
| Surface Tension (at melting point) | 180 mN/m |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 20°C) | 4.7 µΩ·m |
| Thermal Conductivity | 142 W/m·K |
| Magnetic Susceptibility | -5 × 10^-6 cm^3/mol |
| Electronegativity (Pauling scale) | 0.93 |
| Property | Value with Unit |
|---|---|
| Atomic Number | 11 |
| Atomic Mass | 22.98976928 amu |
| Isotopes | ^23Na (100%) |
| Nuclear Spin (for ^23Na) | 3/2 ℏ |
| Neutron Cross Section (for ^23Na) | 0.53 barns |
| Nuclear Magnetic Moment (for ^23Na) | 2.2175 µN |
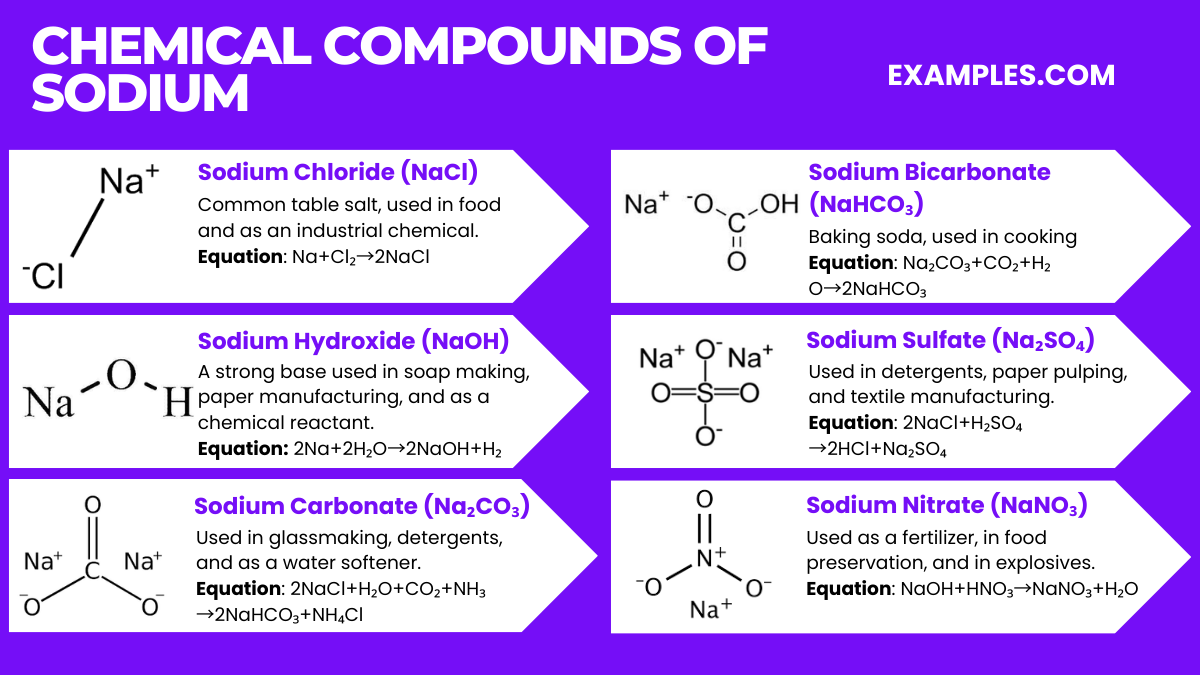
Sodium forms a range of important chemical compounds, each with distinct applications. Here are six key sodium compounds along with their chemical equations:
Sodium has several isotopes, each with specific characteristics. The table below provides an overview:
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Decay Mode |
|---|---|---|---|---|
| Na-22 | 22 | Synthetic | 2.6 years | Beta decay |
| Na-23 | 23 | 100 | Stable | – |
| Na-24 | 24 | Synthetic | 15 hours | Beta decay |
The most common and naturally occurring isotope is Sodium-23, which constitutes all naturally occurring sodium. The other isotopes, like Sodium-22 and Sodium-24, are artificially produced and have applications in medical and scientific research, particularly in nuclear medicine and as tracers.
Sodium has various applications due to its chemical properties. Here are five of its most significant uses:
Sodium is primarily produced through the electrolysis of sodium chloride (table salt), a process known as the Downs process:
Sodium, an essential nutrient, has significant impacts on human health, both beneficial and potentially harmful:
The environmental impact of sodium is primarily related to its compounds and industrial usage:
Sodium regulates blood pressure and fluid balance, supports nerve function and muscle contraction, but excessive intake can lead to health issues like hypertension.
Sodium is a soft, silvery-white metal, highly reactive and a key component of table salt (sodium chloride) when combined with chlorine.
Sodium is used in table salt, industrial chemicals (like caustic soda), street lighting (sodium vapor lamps), and as a heat conductor in some nuclear reactors.
Symptoms of excessive sodium intake include high blood pressure, swelling or edema, headache, dehydration, and in severe cases, heart and kidney problems.
Sodium, a vital element in both biology and industry, plays key roles ranging from regulating bodily functions to industrial applications. However, its management, both in dietary intake and environmental impact, is crucial. Understanding sodium’s properties, uses, and health implications empowers educators to teach its significance effectively, emphasizing the importance of balance in its consumption and usage.

Sodium, an essential alkali metal, plays a pivotal role in both the scientific world and our daily lives. This comprehensive guide delves into the fundamental aspects of sodium, exploring its properties, diverse applications, and critical safety tips. Perfect for teachers, it provides practical examples to enrich classroom discussions, from its role in the human body to its use in industries. Understanding sodium’s dynamics enhances educational experiences, making science both relatable and intriguing.
Sodium is a highly reactive, silvery-white alkali metal, known for its abundance in the Earth’s crust and oceans. It’s essential for various biological functions and is widely used in compounds like sodium chloride (table salt). In its pure form, sodium reacts swiftly with water and air, necessitating careful handling. Its properties and compounds make it a fundamental element in both chemistry education and various industrial applications.
Formula: Na
Composition: A single sodium atom.
Bond Type: Highly reactive, especially with water.
Molecular Structure: Soft metal.
Electron Configuration: 11 electrons; configuration 1s² 2s² 2p⁶ 3s¹.
Significance: Essential in daily life, used in table salt and industrial applications.
Role in Chemistry: Forms vital compounds like sodium chloride (NaCl).


Physical Property | Description |
|---|---|
Color | Silvery-white |
State at Room Temperature | Solid |
Density | About 0.968 g/cm³ |
Melting Point | 97.79°C (208°F) |
Boiling Point | 883°C (1621°F) |
Hardness | Soft, can be cut with a knife |
Conductivity | Good conductor of heat and electricity |
Luster | Metallic when fresh; tarnishes quickly in air due to oxidation |
Sodium exhibits several notable chemical properties:
High Reactivity: Sodium is highly reactive, particularly with water. It reacts vigorously, producing sodium hydroxide and hydrogen gas.
Equation: 2Na+2H₂O→2NaOH+H₂
Atomic Number: Sodium has an atomic number of 11, placing it in the alkali metals group in the periodic table.
Electronegativity: With an electronegativity of 0.93 on the Pauling scale, sodium tends to lose its one valence electron to form ionic compounds.
Flammability: When exposed to air, sodium can ignite, burning to form sodium oxide.
Equation: 4Na+O₂→2Na₂O
Valence Electrons: Sodium has a single electron in its outermost shell, making it highly reactive and prone to forming cations (Na⁺).
Compound Formation: Sodium forms a variety of compounds, notably sodium chloride (NaCl), sodium hydroxide (NaOH), and sodium carbonate (Na₂CO₃).
Sodium Chloride: Na+Cl₂→2NaCl
Sodium Hydroxide: 2Na+2H₂O→2NaOH+H₂
Sodium Carbonate: 2NaOH+CO₂→Na₂CO₃+H₂O
Position in Periodic Table: As a member of Group 1, the alkali metals, sodium is characterized by its high reactivity and tendency to form +1 oxidation states.
Reaction with Oxygen: In the presence of oxygen, sodium forms oxides and peroxides.
Equation: 2Na+O₂→Na₂O₂ (Sodium Peroxide)
Property | Value with Unit |
|---|---|
Boiling Point | 883 °C |
Melting Point | 97.79 °C |
Critical Temperature | 2573 °C |
Critical Pressure | 35 MPa |
Heat of Vaporization | 97.42 kJ/mol |
Heat of Fusion | 2.60 kJ/mol |
Specific Heat Capacity (at 25°C) | 1.23 J/g·K |
Thermal Conductivity | 142 W/m·K |
Property | Value with Unit |
|---|---|
Density (at 20°C) | 968 kg/m³ |
Viscosity (at 100°C) | 0.71 mPa·s |
Solubility in Water | Reacts with water |
Refractive Index | NA (Metallic) |
Surface Tension (at melting point) | 180 mN/m |
Property | Value with Unit |
|---|---|
Electrical Resistivity (at 20°C) | 4.7 µΩ·m |
Thermal Conductivity | 142 W/m·K |
Magnetic Susceptibility | -5 × 10^-6 cm^3/mol |
Electronegativity (Pauling scale) | 0.93 |
Property | Value with Unit |
|---|---|
Atomic Number | 11 |
Atomic Mass | 22.98976928 amu |
Isotopes | ^23Na (100%) |
Nuclear Spin (for ^23Na) | 3/2 ℏ |
Neutron Cross Section (for ^23Na) | 0.53 barns |
Nuclear Magnetic Moment (for ^23Na) | 2.2175 µN |

Sodium forms a range of important chemical compounds, each with distinct applications. Here are six key sodium compounds along with their chemical equations:
Sodium Chloride (NaCl)
Equation: Na+Cl₂→2NaCl
Common table salt, used in food and as an industrial chemical.
Sodium Hydroxide (NaOH)
Equation: 2Na+2H₂O→2NaOH+H₂
A strong base used in soap making, paper manufacturing, and as a chemical reactant.
Sodium Carbonate (Na₂CO₃)
Equation: 2NaCl+H₂O+CO₂+NH₃→2NaHCO₃+NH₄Cl
Known as soda ash, used in glassmaking, detergents, and as a water softener.
Sodium Bicarbonate (NaHCO₃)
Equation: Na₂CO₃+CO₂+H₂O→2NaHCO₃
Baking soda, used in cooking, as a cleaning agent, and in antacids.
Sodium Sulfate (Na₂SO₄)
Equation: 2NaCl+H₂SO₄→2HCl+Na₂SO₄
Used in detergents, paper pulping, and textile manufacturing.
Sodium Nitrate (NaNO₃)
Equation: NaOH+HNO₃→NaNO₃+H₂O
Used as a fertilizer, in food preservation, and in explosives.
Sodium has several isotopes, each with specific characteristics. The table below provides an overview:
Isotope | Mass Number | Natural Abundance (%) | Half-Life | Decay Mode |
|---|---|---|---|---|
Na-22 | 22 | Synthetic | 2.6 years | Beta decay |
Na-23 | 23 | 100 | Stable | – |
Na-24 | 24 | Synthetic | 15 hours | Beta decay |
The most common and naturally occurring isotope is Sodium-23, which constitutes all naturally occurring sodium. The other isotopes, like Sodium-22 and Sodium-24, are artificially produced and have applications in medical and scientific research, particularly in nuclear medicine and as tracers.
Sodium has various applications due to its chemical properties. Here are five of its most significant uses:
Sodium Chloride (Table Salt): Sodium chloride, commonly known as table salt, is extensively used in food seasoning and preservation. It’s also crucial in industrial processes, like the manufacture of chlorine and caustic soda.
Sodium Hydroxide (Caustic Soda): Sodium hydroxide is used in the production of soaps, paper, and synthetic fibers like rayon. It’s also employed in water treatment and as a cleaning agent in various industries.
Sodium Bicarbonate (Baking Soda): Widely used in baking as a leavening agent, sodium bicarbonate also finds applications in cleaning, fire extinguishers, and antacids due to its neutralizing properties.
Sodium Carbonate (Soda Ash): This compound is essential in glass manufacturing, where it reduces the melting temperature of silica. It’s also used in detergents and as a water softener.
Street Lights and Lamps: Sodium vapor lamps, which emit a characteristic yellow light, are used for street lighting. They are energy efficient and have a longer lifetime compared to traditional incandescent bulbs.
Sodium is primarily produced through the electrolysis of sodium chloride (table salt), a process known as the Downs process:
Raw Material: The primary raw material for sodium production is sodium chloride, which can be obtained from salt mines or through the evaporation of seawater.
Electrolysis Process: In the Downs process, molten sodium chloride is electrolyzed using a cathode and an anode in a specially designed cell. At the cathode, sodium ions gain electrons and form liquid sodium.
Collection and Purification: The liquid sodium is collected and often undergoes further purification to remove impurities, ensuring it meets quality standards for various applications.
Energy-Intensive Process: The production of sodium is energy-intensive, requiring high temperatures to melt the salt and significant electrical energy for electrolysis.
Chlorine Co-Product: Chlorine gas is a by-product of this process, which itself is an important chemical used in various industrial applications.
Sodium, an essential nutrient, has significant impacts on human health, both beneficial and potentially harmful:
Blood Pressure Regulation: Sodium plays a crucial role in maintaining blood pressure and fluid balance in the body. However, excessive sodium intake can lead to high blood pressure, a risk factor for heart disease and stroke.
Hyponatremia: Low levels of sodium in the blood, known as hyponatremia, can occur due to excessive water intake, certain medications, or diseases. Symptoms include headache, confusion, seizures, and in severe cases, coma.
Heart Health: High sodium consumption is linked to an increased risk of heart disease. It can lead to hypertension and strain on the heart, contributing to heart failure over time.
Kidney Function: Excess sodium can affect kidney function, as the kidneys work to filter and eliminate excess sodium. Chronic high sodium intake can lead to kidney stones and kidney disease.
Bone Health: High sodium intake is associated with calcium loss, which can lead to weakened bones and conditions like osteoporosis.
Dietary Recommendations: Health organizations recommend moderating sodium intake to reduce health risks. This includes consuming more natural foods and less processed foods, which often contain high levels of sodium.
The environmental impact of sodium is primarily related to its compounds and industrial usage:
Soil Salinity: Excessive use of sodium-based compounds, like sodium chloride for de-icing roads, can lead to increased soil salinity. This affects soil quality and plant growth.
Water Pollution: Sodium compounds can contaminate water bodies, affecting aquatic life. High sodium levels can alter water density, impacting aquatic ecosystems.
Sodium in Agriculture: Sodium-containing fertilizers and pesticides can accumulate in the soil, potentially affecting soil health and crop yields.
Industrial Discharge: Industries that use sodium compounds may discharge excess sodium into water systems, affecting water quality and aquatic organisms.
Air Quality: Some sodium compounds, when released into the atmosphere, can contribute to air pollution. For example, sodium vapor lamps can release small amounts of sodium into the air.
Waste Management: Disposal of sodium-containing waste needs to be managed properly to prevent environmental contamination.
Sodium regulates blood pressure and fluid balance, supports nerve function and muscle contraction, but excessive intake can lead to health issues like hypertension.
Sodium is a soft, silvery-white metal, highly reactive and a key component of table salt (sodium chloride) when combined with chlorine.
Sodium is used in table salt, industrial chemicals (like caustic soda), street lighting (sodium vapor lamps), and as a heat conductor in some nuclear reactors.
Symptoms of excessive sodium intake include high blood pressure, swelling or edema, headache, dehydration, and in severe cases, heart and kidney problems.
Sodium, a vital element in both biology and industry, plays key roles ranging from regulating bodily functions to industrial applications. However, its management, both in dietary intake and environmental impact, is crucial. Understanding sodium’s properties, uses, and health implications empowers educators to teach its significance effectively, emphasizing the importance of balance in its consumption and usage.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Sodium?
10
11
12
13
Which of the following properties is NOT characteristic of sodium?
Silver-colored
Highly reactive
Heavy and dense
Soft enough to cut with a knife
Sodium is essential in the human body primarily for:
Bone formation
Nerve impulse transmission
Muscle contraction
Both B and C
What compound does sodium form when it reacts with water?
Sodium hydroxide
Sodium chloride
Sodium oxide
Sodium peroxide
Which industry primarily uses sodium in large amounts?
Textile
Paper
Glass making
Petroleum
The natural source of sodium is primarily:
Fresh water
Rocks and minerals
Plant materials
Atmospheric gases
Sodium vapor lamps emit light that is primarily:
Red
Blue
Yellow
Green
A common dietary source of sodium is:
Fresh fruits
Red meat
Table salt
Whole grains
Sodium's role in food preservation primarily involves:
Enhancing flavor
Absorbing moisture
Preventing microbial growth
All of the above
In terms of reactivity, sodium:
Does not react with oxygen
Reacts violently with water
Is stable at high temperatures
Cannot form compounds with non-metals
Before you leave, take our quick quiz to enhance your learning!

