Which of the following properties is true about tantalum?
Highly reactive
Poor conductor of electricity
Corrosion-resistant
Low melting point
Dive into the compelling world of Tantalum, an element shrouded in both mystery and practicality. This robust guide unfolds Tantalum’s definition, unpacks its meaning, and illuminates its myriad uses and compounds. Tantalum is more than just a metal; it’s a symbol of human progress, deeply interwoven with technological advances, from enhancing electronics to revolutionizing medicine. Here, we explore this tantalizing element, revealing its role as an unsung hero in applications that touch our everyday lives. Our journey through the properties and applications of Tantalum is enriched with examples that showcase its versatility and indispensable nature in the modern world.
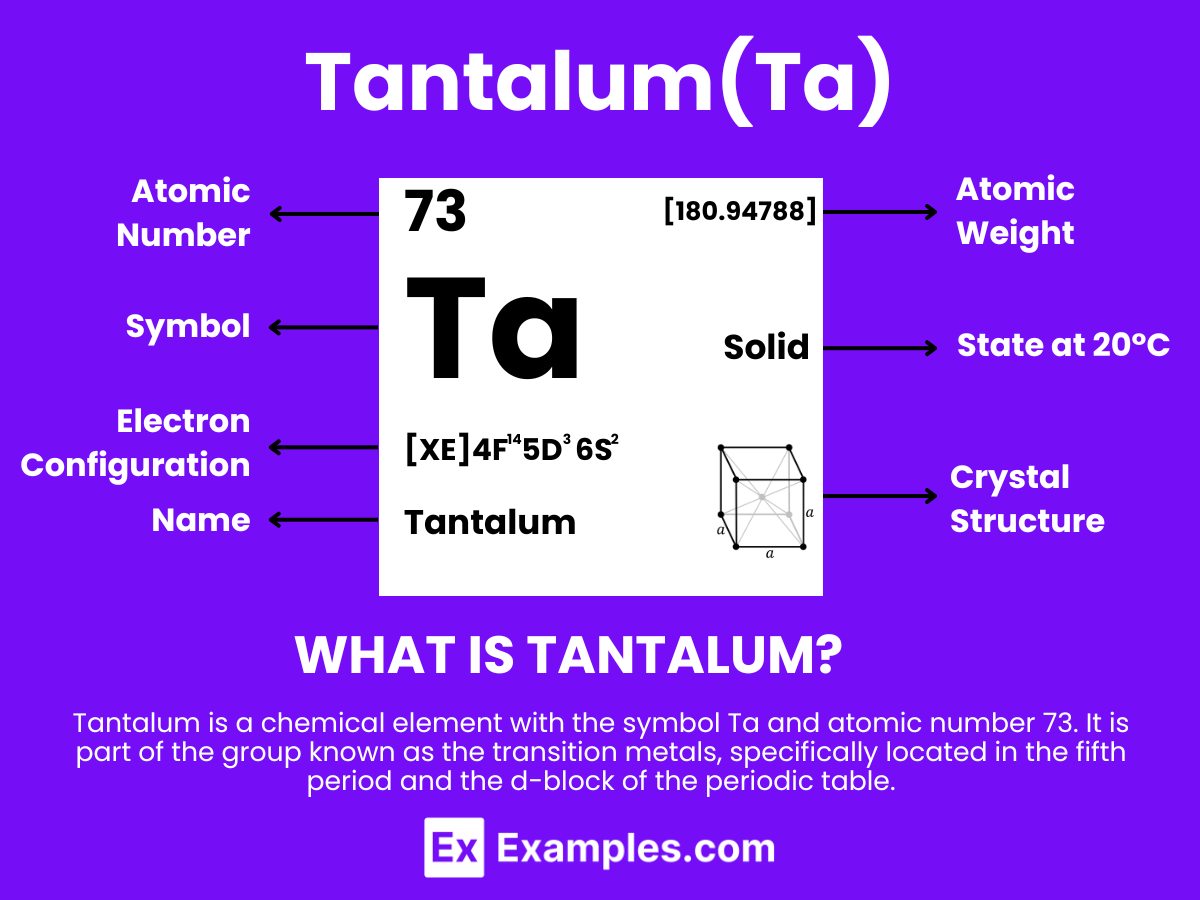
Tantalum is a metallic element with the chemical symbol Ta and atomic number 73. It is extracted from tantalite minerals, where it commonly occurs alongside niobium, due to their similar chemical properties. Tantalum exists naturally and is distinguished by its high melting point and exceptional resistance to corrosion, which make it invaluable in various industrial and medical applications. The discovery of tantalum was crucial in the field of chemistry for its contribution to the understanding of the periodic table’s transition metals. Thanks to its ability to form extremely stable and thin oxide layers, tantalum is pivotal in electronic components.
Delving into the atomic structure of Tantalum reveals the foundation of its remarkable properties and wide-ranging applications. Housing 73 protons in its nucleus, Tantalum’s atomic attributes are a gateway to understanding its role in advanced technologies.
The stability and behavior of Tantalum under varying temperatures and pressures have been thoroughly investigated, revealing that it remains solid under standard conditions. Its high melting point and robustness at high temperatures underscore its importance in demanding applications like electronic devices, aerospace technology, and surgical implants, reflecting Tantalum’s pivotal role in pushing the boundaries of modern science and technology.
| Property | Value |
| Appearance | Shiny, bluish-gray, lustrous metallic |
| Atomic Number | 73 |
| Atomic Mass | 180.94788 amu |
| State at 20 °C | Solid |
| Melting Point | 3017 °C |
| Boiling Point | 5458 °C |
| Density | 16.69 g/cm³ |
| Electron Configuration | [Xe]4f¹⁴ 5d³ 6s² |
| Oxidation States | +5 (most stable), possible +2, +3, +4 |
| Crystal Structure | Body-centered cubic (bcc) |
Tantalum’s chemical properties are characterized by its exceptional resistance to corrosion and high melting point, distinguishing it from many other metals.
| Property | Value |
|---|---|
| State at 20 °C | Solid |
| Melting Point | 3017 °C |
| Boiling Point | 5458 °C |
| Density | 16.69 g/cm³ |
| Specific Heat | 140 J/(kg·K) |
| Thermal Conductivity | 57 W/(m·K) |
| Property | Value |
|---|---|
| Appearance | Shiny and silvery |
| Crystal Structure | Body-centered cubic (bcc) |
| Hardness | Vickers: 870–1200 HV |
| Elastic Modulus | 186 GPa |
| Poisson’s Ratio | 0.34 |
| Property | Value |
|---|---|
| Electrical Resistivity | 131 nΩ·m (at 20 °C) |
| Magnetic Ordering | Paramagnetic |
| Property | Value |
|---|---|
| Atomic Number | 73 |
| Atomic Mass | 180.94788 u |
| Isotopes | ¹⁸⁰Ta (naturally occurring, stable isotope) among others |
| Radioactivity | ¹⁸⁰Ta is the only naturally occurring stable isotope, which is also considered to have a very long half-life, making it quasi-stable. Other isotopes are synthetic and have varying degrees of radioactivity. |
Coltan Separation: Tantalum is primarily extracted from the mineral coltan (columbite-tantalite), where it is found alongside niobium. The refinement process involves separating tantalum from niobium and other minerals due to their different chemical properties.
Liquid-Liquid Extraction: The separation of tantalum from niobium often utilizes liquid-liquid extraction methods, using organic solvents that selectively dissolve tantalum compounds.
Fluoride Reduction: Tantalum is purified through the reduction of potassium (K2TaF7), typically using metallic sodium in a reaction that yields pure tantalum metal.
Reduction Techniques: Pure tantalum can also be obtained by reducing tantalum pentachloride (TaCl5) or tantalum oxide (Ta2O5) with a reductant such as metallic sodium or magnesium.
Tantalum forms oxides, notably tantalum pentoxide (Ta₂O₅), used in electronic capacitors for its high dielectric constant and stability.
Equation: 2Ta + 5O₂ → 2Ta₂O₅
Tantalum pentafluoride (TaF₅) is produced in reactions involving tantalum and fluorine, showcasing tantalum’s ability to form compounds in a +5 oxidation state.
Equation: 2Ta + 5F₂ → 2TaF₅
Tantalum pentachloride (TaCl₅) is synthesized through the direct chlorination of tantalum, illustrating its reactivity with halogens.
Equation: 2Ta + 5Cl₂ → 2TaCl₅
Tantalum pentaiodide (TaI₅) can be produced, indicating tantalum’s potential to achieve a +5 oxidation state with iodine.
Equation: 2Ta + 5I₂ → 2TaI₅
In aqueous solutions, tantalum can form various ions and complexes, highlighting its chemical versatility.
Equation: Ta → Ta5⁺ + 5e⁻
Tantalum forms complex ions with different ligands, underlining its role in coordination chemistry.
Equation: Ta5⁺ + nL → [TaLn]5⁺
| Isotope | Half-life | Mode of Decay |
|---|---|---|
| Tantalum-253 | 0.6 seconds | Alpha decay |
| Tantalum-254 | 13 seconds | Alpha decay |
| Tantalum-255 | 21.5 seconds | Alpha decay |
| Tantalum-256 | 27 seconds | Alpha decay |
| Tantalum-257 | 0.65 seconds | Alpha decay |
| Tantalum-258 | 4.1 seconds | Alpha decay |
| Tantalum-259 | 6.2 seconds | Alpha decay |
| Tantalum-260 | 2.7 minutes | Alpha decay |
Nuclear Industry: While not commonly used for neutron absorption like hafnium, tantalum’s heat resistance and durability can serve specific roles in nuclear applications, ensuring reliability under extreme conditions.
Aerospace Components: Tantalum’s high melting point and resistance to thermal shock make it essential for critical aerospace materials, including components for rocket nozzles and heat shields.
Electronic Capacitors: Tantalum’s ability to form stable oxide layers makes tantalum capacitors an industry standard for reliability, particularly in automotive and portable electronics.
Specialty Alloys: The addition of tantalum to alloys enhances strength, ductility, and corrosion resistance, making these materials suitable for a variety of demanding industrial applications.
Scientific Exploration: Tantalum is at the forefront of scientific exploration, particularly in studying superconducting materials and investigating novel alloy compositions.
Technology Development: Tantalum’s role in the advancement of technology, especially in electronics, is critical, with ongoing development in areas such as microprocessors and high-performance computing.
This detailed outline presents Tantalum as a highly versatile element, essential to various high-tech industries due to its unique attributes. Tantalum’s ability to withstand extreme conditions and resist corrosion makes it invaluable in nuclear reactors, aerospace, and the semiconductor industry, while its biocompatibility has opened doors in medical technology. The exploration and usage of Tantalum continue to advance our scientific understanding and technological capabilities.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
Which of the following properties is true about tantalum?
Highly reactive
Poor conductor of electricity
Corrosion-resistant
Low melting point
What is the primary use of tantalum in electronics?
Conductors
Capacitors
Resistors
Transistors
Which mineral is the main source of tantalum?
Bauxite
Cassiterite
Columbite-tantalite
Hematite
What is the melting point of tantalum?
2468°C
3020°C
3290°C
3422°C
Tantalum is most commonly found in which type of geological deposits?
Sedimentary
Igneous
Metamorphic
Alluvial
Which country is one of the largest producers of tantalum?
Australia
Russia
Brazil
China
What is the density of tantalum?
10.2 g/cm³
16.6 g/cm³
19.3 g/cm³
21.4 g/cm³
In which year was tantalum discovered?
1802
1824
1846
1867
What color does tantalum exhibit in its pure form?
Silver-gray
Gold
Black
White
Which chemical property makes tantalum suitable for surgical implants?
High reactivity
Low density
Biocompatibility
Radioactivity
Before you leave, take our quick quiz to enhance your learning!

