What is the atomic number of Thallium?
80
81
82
83
Thallium, a lesser-known yet fascinating element, presents unique opportunities for exploration in educational settings. This guide introduces Thallium, shedding light on its chemical properties, real-world applications, and relevance in various scientific contexts. Teachers will find valuable examples illustrating Thallium’s role in chemistry and environmental studies, providing a rich, engaging learning experience for students. Emphasizing safety and environmental impacts, this guide is an excellent resource for educators looking to broaden their teaching horizons with Thallium.
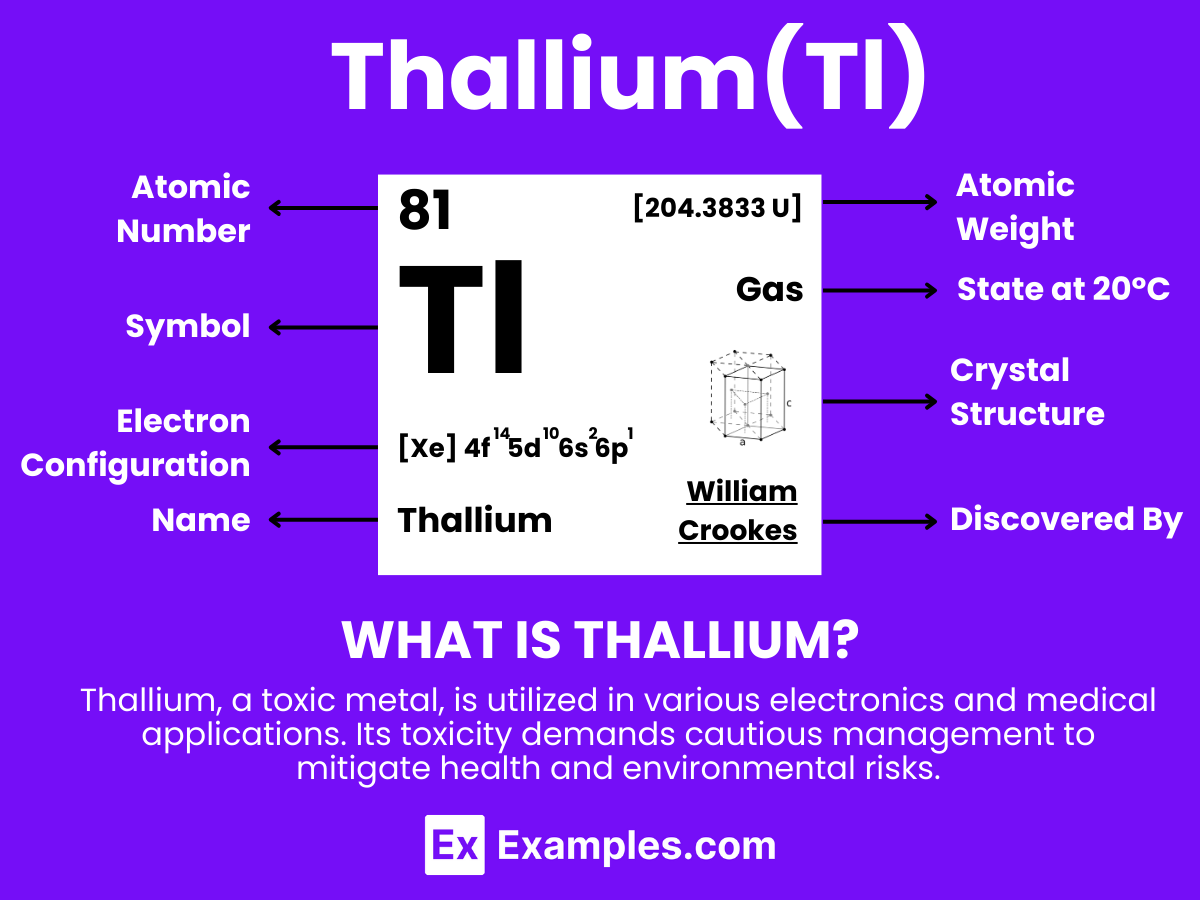
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray, post-transition metal that is not found free in nature.This soft, malleable element resembles tin but discolors when exposed to air. Thallium is used in electronic devices, optical lenses, and some medical procedures. In the classroom, it serves as an intriguing example of physical and chemical properties, as well as a discussion point for its environmental and health effects. Due to its toxicity, Thallium also offers a compelling case study in safety and ethics in science.
Thallium metal (Tl) consists of atoms bonded together. Each thallium atom has 81 protons in its nucleus and varying numbers of neutrons, depending on the isotope. In the thallium solid, the atoms are closely packed in a metallic crystal lattice structure.
Atomic Level: Each thallium atom (Tl) consists of 81 protons and a varying number of neutrons. Metallic Bonding: The thallium atoms form metallic bonds with neighboring atoms, where outer electrons are delocalized and free to move throughout the metal lattice.
The bonding between the thallium atoms is relatively strong due to metallic bonding, resulting in a solid metal with characteristic properties such as malleability, ductility, and conductivity. At room temperature, thallium is a soft, silvery-gray metal with a low melting point, making it useful in various applications such as electronics, high-density optical systems, and medical imaging
| Property | Description |
|---|---|
| Appearance | Soft, silvery-gray metal |
| Atomic Mass | Average atomic mass of approximately 204.38 u |
| Density | Approximately 11.85 g/cm³ at room temperature |
| Melting Point | 304°C (579°F) |
| Boiling Point | 1473°C (2683°F) |
| Electrical Conductivity | Good conductor of electricity |
| Thermal Conductivity | Moderate; about 46.1 W/(m·K) |
| Malleability and Ductility | Highly malleable and ductile; can be cut with a knife |
| Crystal Structure | Hexagonal close-packed (hcp) |
Thallium exhibits a range of chemical properties due to its position in the periodic table. Here’s a detailed look at some of the key chemical properties of thallium, including relevant chemical equations.
Thallium predominantly exists in two oxidation states: +1 (thallium(I) or Tl+) and +3 (thallium(III) or Tl^3+). Thallium(I) compounds are more stable and resemble those of other group 1 elements, while thallium(III) compounds are more oxidative.
Thallium reacts with oxygen to form thallium(I) oxide (Tl2O) or thallium(III) oxide (Tl2O3), depending on the conditions:
Thallium(I) reacts slowly with water, forming thallium(I) hydroxide (TlOH) and hydrogen gas:
Thallium reacts with acids to form corresponding thallium salts and hydrogen gas, similar to the reaction of other metals with acids:
Thallium combines directly with halogens to form thallium(I) or thallium(III) halides:
Thallium(I) compounds, like thallium(I) chloride (TlCl), are soluble in water, which is characteristic of thallium(I) salts. However, thallium(III) compounds are less soluble and more reactive than their +1 counterparts.
Both thallium(I) and thallium(III) compounds are highly toxic, and thallium salts can be absorbed through the skin. Thallium’s toxicity is a significant concern in its chemical handling and applications.
The preparation of thallium primarily involves the processing of minerals that contain thallium, with thallium being most commonly found in association with zinc and lead ores. The two main methods for extracting thallium are the pyrometallurgical process and the electrolytic process. Here’s a brief overview of each method:
Pyrometallurgical Process: This process involves the thermal treatment of thallium-bearing ores, usually as a byproduct of smelting zinc or lead ores.
Electrolytic Process: In this process, thallium is extracted from its ores through electrolysis, a method more commonly used when thallium is present in solution. The steps include:
Purification and Refining: Following extraction by either the pyrometallurgical or electrolytic process, thallium may require further purification to achieve the desired purity for industrial or commercial use. This can involve additional electrolytic refining or distillation under vacuum, processes that help remove impurities and produce high-purity thallium
Thallium, a chemical element with the symbol Tl and atomic number 81, is known for its softness and malleability. This post-transition metal is not found free in nature and is extracted from pyrite ores and other sulfide minerals. Thallium forms a variety of compounds, mainly in the +1 and +3 oxidation states. Here, we delve into the chemical compounds of thallium, highlighting their properties, formation, and significant equations.
Safety and Environmental AspectsThallium compounds are highly toxic and require careful handling and disposal. Exposure can lead to serious health issues, including thallium poisoning, which affects the nervous system, kidneys, and heart
Thallium, a metal known for its softness and malleability, has several specialized applications due to its unique physical and chemical properties. Despite its toxicity, thallium is used in various industries with careful handling protocols. Here are some of the primary uses of thallium:
Thallium, a versatile metal with the atomic number 81, boasts a rich isotopic landscape that encompasses both stable and radioactive variants. Its isotopes play crucial roles in scientific research, medical applications, and even environmental studies. In this article, we explore the isotopes of thallium, highlighting their properties, occurrences, and uses in various fields.
Thallium has two naturally occurring stable isotopes: Thallium-203 (Tl-203) and Thallium-205 (Tl-205). These isotopes have distinct nuclear properties and abundances in nature.
Beyond the stable varieties, thallium has numerous radioactive isotopes, ranging from Thallium-176 (Tl-176) to Thallium-210 (Tl-210). Among these, Thallium-204 (Tl-204) is noteworthy due to its relatively longer half-life and its use in scientific research.
Thallium isotopes find applications in a diverse set of fields, illustrating the broad impact of these atomic variants.
While the stable isotopes of thallium pose minimal risk, the radioactive isotopes require careful handling and strict safety protocols to mitigate exposure risks. Their use is regulated in medical, research, and industrial applications to ensure the protection of public health and the environment
Thallium is a metal with unique properties and applications, ranging from electronic components to medical imaging. Despite its toxicity, thallium’s utility in various industrial and scientific fields necessitates its commercial production. This article delves into the methods and sources for the commercial production of thallium, highlighting the processes involved from ore extraction to the final purification.
Thallium is not found in its elemental form in nature. It is typically sourced as a by-product from the smelting of other metals, particularly zinc, lead, and copper ores. These ores contain trace amounts of thallium, which can be extracted during the metal refining process.
The commercial production of thallium involves several key steps, starting from the extraction of thallium from smelting by-products to its final purification.
During the smelting of zinc, lead, and copper ores, thallium-rich dust and residues are collected. These materials serve as the primary raw material for thallium production.
The collected materials undergo a leaching process, often with a solution of sulfuric acid or sodium hydroxide, to dissolve the thallium while leaving behind the bulk of other materials. This process converts thallium into a soluble form, typically as thallium sulfate or thallate, depending on the leaching agent used.
The thallium is then precipitated from the solution, often by adjusting the pH or adding specific reagents that cause thallium to fall out of solution as a precipitate. The precipitated thallium is collected and subjected to further purification processes to remove impurities. This may involve additional leaching, precipitation, and even electrolysis to refine the thallium to the desired purity.
The final product of the commercial thallium production process is typically thallium in its metallic form or as a compound such as thallium(I) sulfate (Tl2SO4), depending on the demand and application. The purity of the final product can vary, but for many applications, especially in electronics and optics, high-purity thallium is required.
Thallium and its compounds have various applications in the commercial and scientific realms. These include:
Given the toxicity of thallium and its compounds, their production, handling, and disposal are subject to stringent regulations to protect human health and the environment. Producers must employ safety measures to minimize exposure and implement waste management strategies to mitigate environmental impact
Thallium is a highly toxic metal that poses significant health risks when humans are exposed to it, whether through inhalation, ingestion, or skin contact. The severity of thallium’s health effects can vary depending on the dose, duration, and route of exposure. Below are some of the primary health effects associated with thallium exposure:
Thallium is a heavy metal with properties that make it useful in various industrial, electronic, and medical applications. However, its presence in the environment can have significant adverse effects on ecosystems and human health. Understanding the environmental effects of thallium is crucial for mitigating its impacts and ensuring the safety of living organisms and the health of ecosystems.
Thallium enters the environment through both natural and anthropogenic sources. Natural sources include the weathering of thallium-bearing minerals and volcanic activity. Anthropogenic sources, however, are more significant contributors to environmental thallium levels. These include:
Thallium can leach into groundwater and surface waters from industrial sites, affecting aquatic ecosystems. Thallium compounds, particularly soluble ones like thallium sulfate, are highly toxic to aquatic life. They can disrupt the physiological processes of aquatic organisms, leading to reduced biodiversity and the death of sensitive species.
Thallium can accumulate in soils near industrial sites, leading to elevated concentrations that can be toxic to plants. Thallium uptake by plants can inhibit growth, interfere with photosynthesis, and cause chlorosis and necrosis. This not only affects plant health but can also enter the food chain, impacting herbivores and, ultimately, higher trophic levels.
Wildlife exposure to thallium occurs mainly through ingestion of contaminated water, soil, or plants. Thallium toxicity can lead to a range of health problems in animals, including organ damage, reproductive failure, and mortality. Ecosystems can be significantly impacted due to the bioaccumulation and biomagnification of thallium, leading to ecological imbalances.
Thallium is a metal with unique applications in electronics, medical imaging, and scientific research due to its distinct physical and chemical properties. However, its high toxicity necessitates stringent handling and exposure controls. The health risks associated with thallium exposure underscore the importance of safety measures and the ongoing search for safer alternatives in its various uses.

Thallium, a lesser-known yet fascinating element, presents unique opportunities for exploration in educational settings. This guide introduces Thallium, shedding light on its chemical properties, real-world applications, and relevance in various scientific contexts. Teachers will find valuable examples illustrating Thallium’s role in chemistry and environmental studies, providing a rich, engaging learning experience for students. Emphasizing safety and environmental impacts, this guide is an excellent resource for educators looking to broaden their teaching horizons with Thallium.
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray, post-transition metal that is not found free in nature.This soft, malleable element resembles tin but discolors when exposed to air. Thallium is used in electronic devices, optical lenses, and some medical procedures. In the classroom, it serves as an intriguing example of physical and chemical properties, as well as a discussion point for its environmental and health effects. Due to its toxicity, Thallium also offers a compelling case study in safety and ethics in science.
Formula: Tl
Composition: A single thallium atom.
Bond Type: Thallium primarily forms covalent bonds, with three valence electrons contributing.
Molecular Structure: Soft, malleable metal with a bluish-white color, displaying a hexagonal close-packed crystal structure.
Electron Configuration: 81 electrons, with the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶ 4f¹⁴ 5d¹⁰ 6s² 6p¹.
Significance: Used in low-temperature thermometers, infrared detectors, and as a glass additive.
Role in Chemistry: Thallium compounds are important in research, particularly in inorganic synthesis and spectroscopy.
Thallium metal (Tl) consists of atoms bonded together. Each thallium atom has 81 protons in its nucleus and varying numbers of neutrons, depending on the isotope. In the thallium solid, the atoms are closely packed in a metallic crystal lattice structure.
Atomic Level: Each thallium atom (Tl) consists of 81 protons and a varying number of neutrons. Metallic Bonding: The thallium atoms form metallic bonds with neighboring atoms, where outer electrons are delocalized and free to move throughout the metal lattice.
The bonding between the thallium atoms is relatively strong due to metallic bonding, resulting in a solid metal with characteristic properties such as malleability, ductility, and conductivity. At room temperature, thallium is a soft, silvery-gray metal with a low melting point, making it useful in various applications such as electronics, high-density optical systems, and medical imaging
Property | Description |
|---|---|
Appearance | Soft, silvery-gray metal |
Atomic Mass | Average atomic mass of approximately 204.38 u |
Density | Approximately 11.85 g/cm³ at room temperature |
Melting Point | 304°C (579°F) |
Boiling Point | 1473°C (2683°F) |
Electrical Conductivity | Good conductor of electricity |
Thermal Conductivity | Moderate; about 46.1 W/(m·K) |
Malleability and Ductility | Highly malleable and ductile; can be cut with a knife |
Crystal Structure | Hexagonal close-packed (hcp) |
Thallium exhibits a range of chemical properties due to its position in the periodic table. Here’s a detailed look at some of the key chemical properties of thallium, including relevant chemical equations.
Thallium predominantly exists in two oxidation states: +1 (thallium(I) or Tl+) and +3 (thallium(III) or Tl^3+). Thallium(I) compounds are more stable and resemble those of other group 1 elements, while thallium(III) compounds are more oxidative.
Thallium reacts with oxygen to form thallium(I) oxide (Tl2O) or thallium(III) oxide (Tl2O3), depending on the conditions:
2Tl+O₂→Tl2O₂ (thallium(I) oxide)
4Tl+₃O₂→2Tl₂O₃ (thallium(III) oxide)
Thallium(I) reacts slowly with water, forming thallium(I) hydroxide (TlOH) and hydrogen gas:
2Tl+2H₂O→2TlOH+H₂
Thallium reacts with acids to form corresponding thallium salts and hydrogen gas, similar to the reaction of other metals with acids:
2Tl+2HCl→2TlCl+H₂ (with hydrochloric acid)
Thallium combines directly with halogens to form thallium(I) or thallium(III) halides:
2Tl+F₂→2TlF₂ (thallium(I) fluoride)
2Tl+3Cl₂→2TlCl₃ (thallium(III) chloride)
Thallium(I) compounds, like thallium(I) chloride (TlCl), are soluble in water, which is characteristic of thallium(I) salts. However, thallium(III) compounds are less soluble and more reactive than their +1 counterparts.
Both thallium(I) and thallium(III) compounds are highly toxic, and thallium salts can be absorbed through the skin. Thallium’s toxicity is a significant concern in its chemical handling and applications.
The preparation of thallium primarily involves the processing of minerals that contain thallium, with thallium being most commonly found in association with zinc and lead ores. The two main methods for extracting thallium are the pyrometallurgical process and the electrolytic process. Here’s a brief overview of each method:
Pyrometallurgical Process: This process involves the thermal treatment of thallium-bearing ores, usually as a byproduct of smelting zinc or lead ores.
Roasting: The ore is heated in the presence of oxygen, which helps to oxidize the thallium present into thallium(I) oxide (Tl₂O).
Reduction: The thallium(I) oxide is then mixed with carbon and heated, reducing the thallium(I) oxide to metallic thallium.
Condensation: Thallium vaporizes at the high temperatures used in the reduction process and is then condensed into liquid thallium, which can be further purified.
Electrolytic Process: In this process, thallium is extracted from its ores through electrolysis, a method more commonly used when thallium is present in solution. The steps include:
Leaching: The thallium-bearing ores are treated with acids or bases to dissolve the thallium, forming a thallium-containing solution.
Electrolysis: The thallium-containing solution undergoes electrolysis. At the cathode, thallium ions are reduced to form metallic thallium.
Purification: The metallic thallium produced is then purified through various processes, including distillation or electrolytic refining, to obtain pure thallium.
Purification and Refining: Following extraction by either the pyrometallurgical or electrolytic process, thallium may require further purification to achieve the desired purity for industrial or commercial use. This can involve additional electrolytic refining or distillation under vacuum, processes that help remove impurities and produce high-purity thallium
Thallium, a chemical element with the symbol Tl and atomic number 81, is known for its softness and malleability. This post-transition metal is not found free in nature and is extracted from pyrite ores and other sulfide minerals. Thallium forms a variety of compounds, mainly in the +1 and +3 oxidation states. Here, we delve into the chemical compounds of thallium, highlighting their properties, formation, and significant equations.
Thallium(I) Oxide (Tl2O)
Equation: 4Tl+O₂→2Tl₂O
Forms by heating thallium in air, yielding a yellow solid.
Thallium(I) Sulfate (Tl2SO4)
Equation: Tl₂O+2H₂SO₄→Tl2SO₄+2H₂O
Produced from thallium(I) oxide and sulfuric acid, highly soluble in water.
Thallium(III) Oxide (Tl2O3)
Equation: 4Tl+3O₂→2Tl2O₃
Obtained by oxidizing thallium in excess air, forms a dark-brown solid.
Thallium(III) Chloride (TlCl3)
Equation: 2Tl+3Cl₂→2TlCl₃
Synthesized by reacting thallium with chlorine gas, decomposes in light.
Thallium(I) Chloride (TlCl)
Equation: Tl+Cl₂→TlCl
Formed by directly combining thallium and chlorine, yielding a white solid.
Thallium Hydride (TlH)
Equation: Tl+H₂→TlH
Produced through the direct reaction of thallium with hydrogen gas, unstable and of research interest
Safety and Environmental AspectsThallium compounds are highly toxic and require careful handling and disposal. Exposure can lead to serious health issues, including thallium poisoning, which affects the nervous system, kidneys, and heart
Thallium, a metal known for its softness and malleability, has several specialized applications due to its unique physical and chemical properties. Despite its toxicity, thallium is used in various industries with careful handling protocols. Here are some of the primary uses of thallium:
Thallium sulfide (Tl_2S) and other thallium compounds are utilized in photoelectric cells. Thallium-doped semiconductors find applications in infrared detectors and other electronic devices due to their ability to alter electrical properties.
Thallium radioisotopes, such as thallium-201, are used in nuclear medicine, specifically in stress tests to diagnose coronary artery disease. These isotopes help in visualizing the flow of blood to heart muscles.
Thallium bromide-iodide crystals are used in infrared detection and imaging equipment due to their effectiveness in transmitting infrared light.
Thallium oxide is added to glass to increase its refractive index, making it valuable in the production of special glasses with high-density and high-refractive-index properties. These glasses are used in optical lenses and prisms.
Thallium alloys, particularly thallium-bismuth, are utilized in low-temperature thermometers for their superconductivity properties at temperatures close to absolute zero.
Historically, thallium sulfate was used as a rodenticide and ant killer. However, due to its high toxicity and the risk of accidental poisoning, its use in pest control has been greatly restricted or banned in many countries.
In scientific research, thallium compounds are studied for their potential use in a wide range of applications, including superconductors and advanced materials science
Thallium, a versatile metal with the atomic number 81, boasts a rich isotopic landscape that encompasses both stable and radioactive variants. Its isotopes play crucial roles in scientific research, medical applications, and even environmental studies. In this article, we explore the isotopes of thallium, highlighting their properties, occurrences, and uses in various fields.
Thallium has two naturally occurring stable isotopes: Thallium-203 (Tl-203) and Thallium-205 (Tl-205). These isotopes have distinct nuclear properties and abundances in nature.
Natural Abundance: Approximately 29.5%
Nuclear Properties: Thallium-203 has 122 neutrons. It is the lighter of the two stable isotopes and plays a significant role in the study of thallium’s chemical behavior.
Natural Abundance: About 70.5%
Nuclear Properties: With 124 neutrons, Thallium-205 is slightly heavier than Tl-203. It is more abundant and extensively used in nuclear magnetic resonance (NMR) studies due to its nuclear spin properties.
Beyond the stable varieties, thallium has numerous radioactive isotopes, ranging from Thallium-176 (Tl-176) to Thallium-210 (Tl-210). Among these, Thallium-204 (Tl-204) is noteworthy due to its relatively longer half-life and its use in scientific research.
Half-Life: Approximately 3.78 years
Decay Mode: Beta decay to Lead-204 (Pb-204)
Uses: Tl-204 is used in radiation detection equipment calibration and environmental tracer studies due to its beta emission properties
Thallium isotopes find applications in a diverse set of fields, illustrating the broad impact of these atomic variants.
Scientific Research: The distinct nuclear properties of thallium isotopes, especially Tl-205, are leveraged in NMR spectroscopy to study molecular structures and dynamics.
Medical Applications: Radioactive thallium isotopes, such as Thallium-201 (Tl-201), are used in nuclear medicine for diagnostic imaging. Tl-201, due to its ability to mimic potassium ions, is utilized in cardiac imaging to assess heart muscle health.
Environmental Studies: Radioactive isotopes of thallium, including Tl-204, serve as tracers to study the movement and distribution of thallium in ecosystems, helping to monitor environmental pollution and understand geochemical processes.
While the stable isotopes of thallium pose minimal risk, the radioactive isotopes require careful handling and strict safety protocols to mitigate exposure risks. Their use is regulated in medical, research, and industrial applications to ensure the protection of public health and the environment
Thallium is a metal with unique properties and applications, ranging from electronic components to medical imaging. Despite its toxicity, thallium’s utility in various industrial and scientific fields necessitates its commercial production. This article delves into the methods and sources for the commercial production of thallium, highlighting the processes involved from ore extraction to the final purification.
Thallium is not found in its elemental form in nature. It is typically sourced as a by-product from the smelting of other metals, particularly zinc, lead, and copper ores. These ores contain trace amounts of thallium, which can be extracted during the metal refining process.
Zinc Ores: Zinc processing plants are primary contributors to thallium production. Thallium is found in the dust collected from the flue gases during the smelting of zinc ores.
Lead and Copper Ores: Similarly, the smelting of lead and copper ores yields thallium as a by-product, recovered from the smelting process residues.
The commercial production of thallium involves several key steps, starting from the extraction of thallium from smelting by-products to its final purification.
During the smelting of zinc, lead, and copper ores, thallium-rich dust and residues are collected. These materials serve as the primary raw material for thallium production.
The collected materials undergo a leaching process, often with a solution of sulfuric acid or sodium hydroxide, to dissolve the thallium while leaving behind the bulk of other materials. This process converts thallium into a soluble form, typically as thallium sulfate or thallate, depending on the leaching agent used.
The thallium is then precipitated from the solution, often by adjusting the pH or adding specific reagents that cause thallium to fall out of solution as a precipitate. The precipitated thallium is collected and subjected to further purification processes to remove impurities. This may involve additional leaching, precipitation, and even electrolysis to refine the thallium to the desired purity.
The final product of the commercial thallium production process is typically thallium in its metallic form or as a compound such as thallium(I) sulfate (Tl2SO4), depending on the demand and application. The purity of the final product can vary, but for many applications, especially in electronics and optics, high-purity thallium is required.
Thallium and its compounds have various applications in the commercial and scientific realms. These include:
Optics: Thallium bromide and iodide are used in infrared detectors.
Electronics: Thallium sulfide’s electrical properties make it useful in photocells.
Medical: Thallium-201, a radioactive isotope, is used in nuclear medicine for cardiac imaging.
Given the toxicity of thallium and its compounds, their production, handling, and disposal are subject to stringent regulations to protect human health and the environment. Producers must employ safety measures to minimize exposure and implement waste management strategies to mitigate environmental impact
Thallium is a highly toxic metal that poses significant health risks when humans are exposed to it, whether through inhalation, ingestion, or skin contact. The severity of thallium’s health effects can vary depending on the dose, duration, and route of exposure. Below are some of the primary health effects associated with thallium exposure:
Gastrointestinal Issues: Symptoms can include abdominal pain, nausea, vomiting, and diarrhea. These are often the first signs of acute thallium poisoning.
Neurological Effects: Thallium can lead to acute neurological symptoms such as numbness, tingling in the extremities, muscle weakness, and in severe cases, seizures or coma.
Cardiovascular Problems: High exposure levels may cause changes in heart rhythm or rate, potentially leading to more severe cardiovascular issues.
Hair Loss: One of the hallmark signs of chronic thallium exposure is the loss of hair, as thallium affects the protein synthesis in hair follicles.
Neurological Damage: Prolonged exposure can result in persistent neurological problems, including memory loss, insomnia, depression, and nerve damage that may lead to paralysis.
Skin Conditions: Dermatological symptoms such as rashes, dermatitis, and in some cases, ulcers, can occur.
Thallium can accumulate in the kidneys and liver, leading to renal failure or liver damage over time due to the toxic effects of the metal on these organs.
Exposure to thallium during pregnancy can pose risks to the developing fetus, potentially leading to developmental abnormalities or miscarriage. Men may experience reduced fertility.
While direct links between thallium exposure and cancer are less clear, the toxic effects of heavy metals on cellular processes can potentially lead to carcinogenic outcomes.
Treatment involves the immediate cessation of exposure, decontamination (if applicable), and the administration of Prussian blue (ferric hexacyanoferrate), which binds to thallium and facilitates its excretion from the body
Thallium is a heavy metal with properties that make it useful in various industrial, electronic, and medical applications. However, its presence in the environment can have significant adverse effects on ecosystems and human health. Understanding the environmental effects of thallium is crucial for mitigating its impacts and ensuring the safety of living organisms and the health of ecosystems.
Thallium enters the environment through both natural and anthropogenic sources. Natural sources include the weathering of thallium-bearing minerals and volcanic activity. Anthropogenic sources, however, are more significant contributors to environmental thallium levels. These include:
Mining and Smelting: The extraction and processing of metals like zinc, copper, and lead can release thallium into the environment.
Coal-burning Power Plants: Coal contains trace amounts of thallium, which can be released into the atmosphere when coal is burned for energy.
Cement Production: The manufacture of cement can also emit thallium into the atmosphere.
Electronic Waste: Improper disposal of electronic devices containing thallium compounds contributes to thallium pollution.
Thallium can leach into groundwater and surface waters from industrial sites, affecting aquatic ecosystems. Thallium compounds, particularly soluble ones like thallium sulfate, are highly toxic to aquatic life. They can disrupt the physiological processes of aquatic organisms, leading to reduced biodiversity and the death of sensitive species.
Thallium can accumulate in soils near industrial sites, leading to elevated concentrations that can be toxic to plants. Thallium uptake by plants can inhibit growth, interfere with photosynthesis, and cause chlorosis and necrosis. This not only affects plant health but can also enter the food chain, impacting herbivores and, ultimately, higher trophic levels.
Wildlife exposure to thallium occurs mainly through ingestion of contaminated water, soil, or plants. Thallium toxicity can lead to a range of health problems in animals, including organ damage, reproductive failure, and mortality. Ecosystems can be significantly impacted due to the bioaccumulation and biomagnification of thallium, leading to ecological imbalances.
Thallium is a metal with unique applications in electronics, medical imaging, and scientific research due to its distinct physical and chemical properties. However, its high toxicity necessitates stringent handling and exposure controls. The health risks associated with thallium exposure underscore the importance of safety measures and the ongoing search for safer alternatives in its various uses.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Thallium?
80
81
82
83
What is the symbol for Thallium on the periodic table?
Tl
Th
Ti
Tm
Thallium belongs to which group in the periodic table?
Group 13
Group 14
Group 15
Group 16
What is the common oxidation state of Thallium?
+2
+1
+3
+4
Which of the following is a primary source of Thallium?
Galena
Pyrite
Sphalerite
All of the above
What is Thallium primarily used for in industry?
Making batteries
Producing thermometers
Manufacturing electronic devices
Creating special glasses
Thallium sulfate is used for which of the following applications?
Insecticide
Herbicide
Rat poison
Fungicide
Which property makes Thallium unique among metals?
High density
Low melting point
High electrical conductivity
Low toxicity
What is the color of Thallium in its pure form?
Silver-gray
Gold
Copper-red
Black
Thallium compounds are used in medical imaging for which type of scans?
X-ray
MRI
CT scans
Nuclear medicine scans
Before you leave, take our quick quiz to enhance your learning!

