What is the primary use of tin in modern industries?
Wiring
Solder
Building construction
Pharmaceuticals
Tin, a key element in the periodic table, holds a special place in both historical and modern contexts. This guide delves into the fascinating world of Tin, offering educators valuable insights and examples to enrich their teaching. Known for its malleability and resistance to corrosion, Tin is more than just a material; it’s a gateway to understanding fundamental concepts in chemistry and physics. By incorporating real-life applications and historical perspectives, this article aims to provide teachers with engaging content that sparks curiosity and learning about Tin.
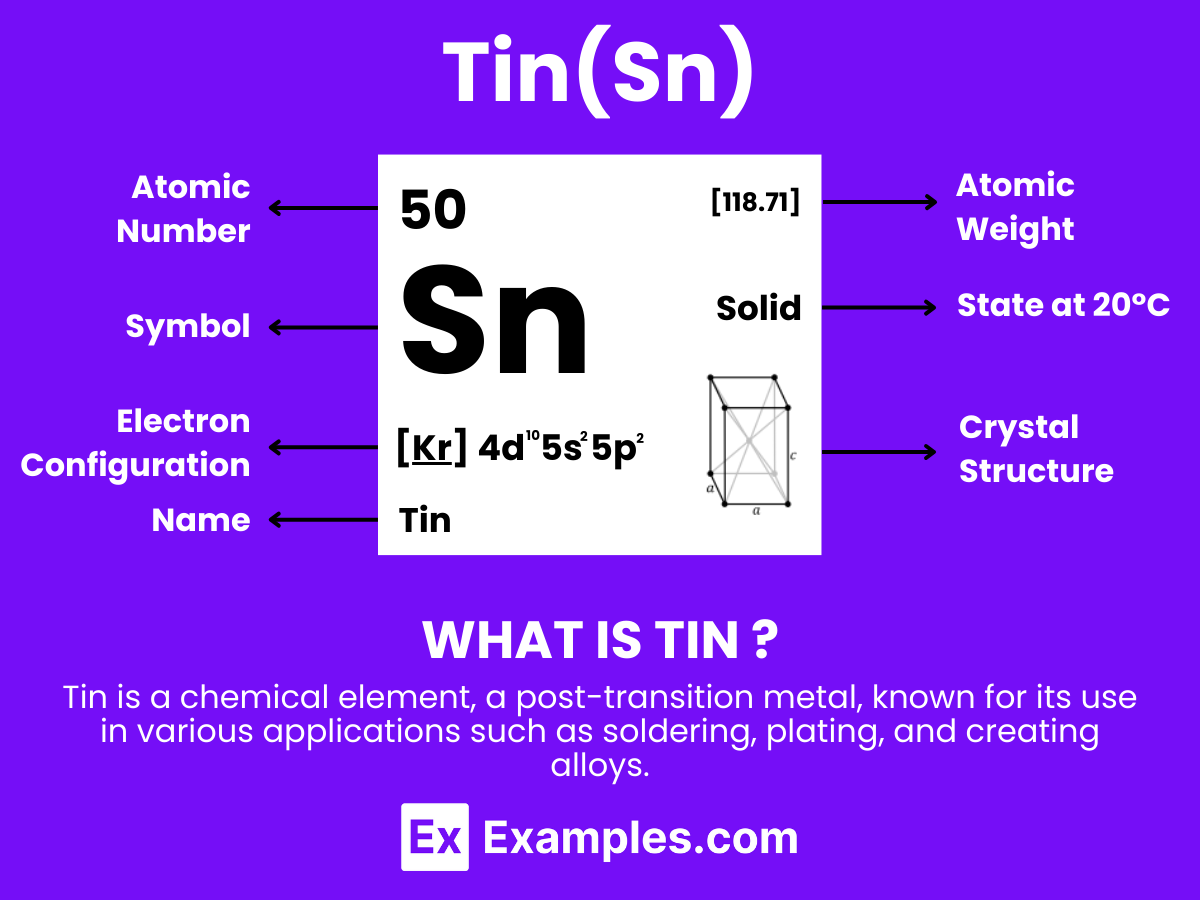
Tin is a chemical element with the symbol Sn (from Latin: stannum) and atomic number 50. It is a post-transition metal known for its silvery-white appearance and high malleability. Tin is not easily oxidized in air, making it resistant to corrosion and ideal for coating other metals. Historically, Tin has been used in alloys such as bronze, and today, it finds applications in solder, tin plating, and various other industries. For teachers, Tin serves as an excellent example to discuss the properties of elements, their place in the periodic table, and their practical applications in everyday life.
Tin (Sn), a chemical element in the carbon group with atomic number 50, exhibits fascinating atomic characteristics that have been pivotal in various technological and industrial applications throughout history. Here’s an overview of the atomic structure of tin:
Neutrons: The number of neutrons in tin can vary, leading to different isotopes of the element. Tin is unique in having a significant number of stable isotopes, the most of any element, with ten stable isotopes ranging from tin-112 to tin-124. These isotopes have varying numbers of neutrons, from 62 to 74, affecting the atomic mass but not the chemical properties of the element.
Electron Shells: Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. The valence electrons (those in the outermost shell) play a crucial role in chemical reactions and the formation of compounds.
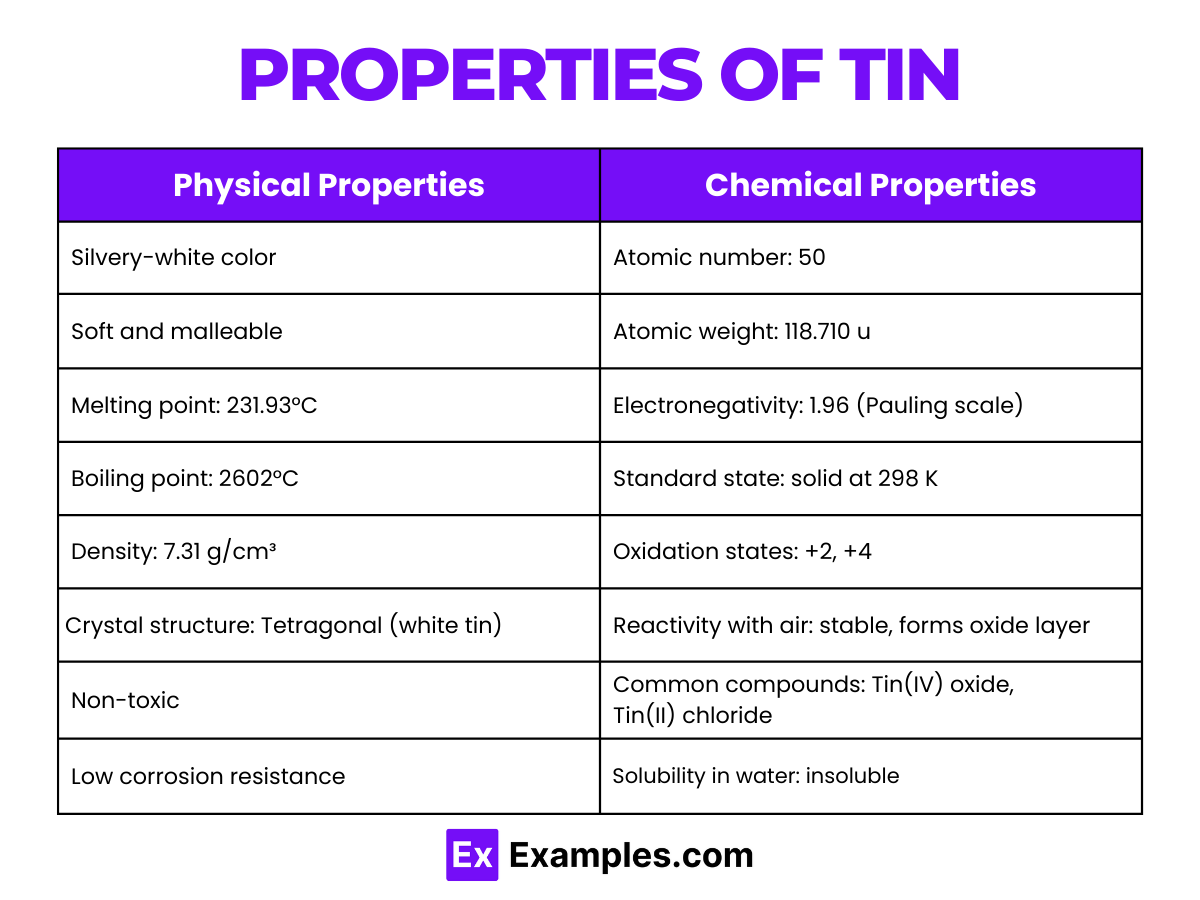
Chemical and Physical Properties: The electron configuration and the distribution of electrons across its shells endow tin with characteristic properties, such as malleability, ductility, and a relatively low melting point for a metal. Tin can exist in two allotropic forms at normal pressure: white tin, which is metallic, and gray tin, which is nonmetallic at temperatures below 13.2°C (55.8°F)
| Property | Description |
|---|---|
| Appearance | Silvery-white, lustrous metal |
| Atomic Mass | Approximately 118.71 u |
| Density | 7.31 g/cm³ at room temperature |
| Melting Point | 231.93°C (449.47°F) |
| Boiling Point | 2602°C (4716°F) |
| Electrical Conductivity | Good conductor of electricity |
| Thermal Conductivity | High thermal conductivity |
| Malleability and Ductility | Highly malleable and ductile |
| Crystal Structure | Tetragonal (white tin) at room temperature; cubic (gray tin) below 13.2°C |
These physical properties underline tin’s utility in various applications, including electronics, coatings, and alloy production.
Tin exhibits a range of chemical behaviors due to its electron configuration and position in the periodic table. Here’s an in-depth look at its chemical properties:
| Property | Value |
|---|---|
| Melting Point | 231.93°C (449.47°F) |
| Boiling Point | 2602°C (4716°F) |
| Heat of Fusion | 7.03 kJ/mol |
| Heat of Vaporization | 296.1 kJ/mol |
| Specific Heat Capacity | 27.112 J/(mol·K) |
| Thermal Conductivity | 66.8 W/(m·K) |
| Property | Value |
|---|---|
| Density | 7.31 g/cm³ |
| Young’s Modulus | 50 GPa |
| Tensile Strength | 14-200 MPa |
| Hardness | 1.5 Mohs |
| Malleability | High |
| Ductility | High |
| Property | Value |
|---|---|
| Electrical Conductivity | 8.69 × 10⁶ S/m |
| Magnetic Susceptibility | -3.8 × 10⁻⁵ |
| Property | Description |
|---|---|
| Atomic Number | 50 – Tin has 50 protons in its nucleus, defining its chemical properties. |
| Atomic Mass | 118.71 u – The average mass of the tin atom, reflecting the sum of its protons and neutrons. |
| Isotopes | Stable: 10 – Tin has the largest number of stable isotopes among all elements, contributing to its diverse applications. |
| Neutron Cross Section | 0.626 barns – A measure of the probability of neutron capture, relevant in nuclear reactions and applications. |
| Half-life of Most Stable Isotope | ^{126}Sn, 2.3 × 10⁵ years – Longest-lived radioactive tin isotope, though most natural tin is stable. |
Tin, symbolized as Sn (from the Latin ‘stannum’), is commonly extracted from its primary ore, cassiterite (SnO₂), through a smelting process that involves several key steps to produce pure tin metal. The preparation of tin can be summarized as follows:
This multi-step process ensures that the tin produced is of high purity, suitable for various industrial and commercial applications.
Tin, a versatile metal, forms numerous compounds exhibiting diverse chemical properties. Here are six significant chemical compounds of tin, along with their descriptions and relevant chemical equations:
Tin is unique among all elements for having the largest number of stable isotopes, with ten recognized by the scientific community. These isotopes range from tin-112 to tin-124, differing in their number of neutrons. Here’s a brief overview of tin’s isotopes:
The diversity of tin’s isotopes is valuable for research in nuclear physics and isotopic geochemistry, providing insights into processes ranging from the formation of solar systems to the behavior of materials under extreme conditions
Tin’s physical and chemical properties make it an essential material in various applications across multiple industries. Here are some of the primary uses of tin:
The commercial production of tin involves several steps, from mining tin ore to refining and processing it into usable forms. The primary source of tin is cassiterite (tin oxide, SnO2), from which tin is extracted through the following processes:
Tin, in its metallic form, is relatively non-toxic and poses little risk to human health. However, certain tin compounds and organotin compounds can have harmful health effects:
Proper handling and industrial hygiene practices are essential to minimize exposure and prevent health risks associated with tin compounds.
Tin’s environmental impact is primarily associated with mining and processing operations. These effects can include:
Tin is called “Sn” because the symbol derives from the Latin name for tin, “stannum.” This nomenclature is part of the international system of chemical symbols used to represent elements based on their Latin names, facilitating universal understanding and communication in the scientific community
Tin is used in a wide array of applications, including soldering materials for electronics, coatings for steel cans to prevent corrosion, glass-making, and creating various alloys like bronze. Its versatility stems from its corrosion resistance, malleability, and low melting point, making it invaluable in industrial and consumer products
Tin is a pure metal in its elemental form, classified under the category of metals in the periodic table. It exists naturally in the Earth’s crust, primarily obtained from the mineral cassiterite (SnO2), and can be processed into a highly pure state for various commercial and industrial applications
In the periodic table, tin is represented by the symbol “Sn” and has an atomic number of 50. It is situated in group 14, also known as the carbon group. This positioning reflects its chemical properties, including the ability to form compounds in multiple oxidation states and its role as a post-transition metal
Tin is a versatile metal with widespread applications in electronics, coatings, and alloys, owing to its distinct physical and chemical properties. While its commercial production is crucial for various industries, attention to the health and environmental impacts of tin and its compounds is essential. Responsible management and recycling can mitigate these effects, sustaining its valuable role in technological advancements and daily use.

Tin, a key element in the periodic table, holds a special place in both historical and modern contexts. This guide delves into the fascinating world of Tin, offering educators valuable insights and examples to enrich their teaching. Known for its malleability and resistance to corrosion, Tin is more than just a material; it’s a gateway to understanding fundamental concepts in chemistry and physics. By incorporating real-life applications and historical perspectives, this article aims to provide teachers with engaging content that sparks curiosity and learning about Tin.
Tin is a chemical element with the symbol Sn (from Latin: stannum) and atomic number 50. It is a post-transition metal known for its silvery-white appearance and high malleability. Tin is not easily oxidized in air, making it resistant to corrosion and ideal for coating other metals. Historically, Tin has been used in alloys such as bronze, and today, it finds applications in solder, tin plating, and various other industries. For teachers, Tin serves as an excellent example to discuss the properties of elements, their place in the periodic table, and their practical applications in everyday life.
Formula: Sn
Composition: A single tin atom.
Bond Type: Tin forms metallic bonds in its metallic form and covalent bonds in its compounds, with four valence electrons.
Molecular Structure: Exists in two main forms – white tin, which is metallic, and gray tin, which is non-metallic.
Electron Configuration: 50 electrons, with the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p².
Significance: Widely used in solder, pewter, and as a protective coating for other metals.
Role in Chemistry: Integral in the study of metal alloys and organometallic chemistry.
Tin (Sn), a chemical element in the carbon group with atomic number 50, exhibits fascinating atomic characteristics that have been pivotal in various technological and industrial applications throughout history. Here’s an overview of the atomic structure of tin:
Protons: Tin has 50 protons in its nucleus, a defining feature that determines its place as element 50 on the periodic table.
Electrons: It also has 50 electrons, with the arrangement in its atomic orbitals following the electron configuration [Kr]4d¹⁰5s² 5p². This configuration influences tin’s chemical behavior and bonding properties.
Neutrons: The number of neutrons in tin can vary, leading to different isotopes of the element. Tin is unique in having a significant number of stable isotopes, the most of any element, with ten stable isotopes ranging from tin-112 to tin-124. These isotopes have varying numbers of neutrons, from 62 to 74, affecting the atomic mass but not the chemical properties of the element.
Stable Isotopes: Include Sn-112, Sn-114 through Sn-120, and Sn-122 through Sn-124. These stable isotopes contribute to tin’s average atomic mass of approximately 118.71 u.
Radioactive Isotopes: Tin also has over 30 unstable isotopes, among which Sn-121 and Sn-123 are used in research and medical applications due to their radioactive properties.
Electron Shells: Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. The valence electrons (those in the outermost shell) play a crucial role in chemical reactions and the formation of compounds.
Chemical and Physical Properties: The electron configuration and the distribution of electrons across its shells endow tin with characteristic properties, such as malleability, ductility, and a relatively low melting point for a metal. Tin can exist in two allotropic forms at normal pressure: white tin, which is metallic, and gray tin, which is nonmetallic at temperatures below 13.2°C (55.8°F)

Property | Description |
|---|---|
Appearance | Silvery-white, lustrous metal |
Atomic Mass | Approximately 118.71 u |
Density | 7.31 g/cm³ at room temperature |
Melting Point | 231.93°C (449.47°F) |
Boiling Point | 2602°C (4716°F) |
Electrical Conductivity | Good conductor of electricity |
Thermal Conductivity | High thermal conductivity |
Malleability and Ductility | Highly malleable and ductile |
Crystal Structure | Tetragonal (white tin) at room temperature; cubic (gray tin) below 13.2°C |
These physical properties underline tin’s utility in various applications, including electronics, coatings, and alloy production.
Tin exhibits a range of chemical behaviors due to its electron configuration and position in the periodic table. Here’s an in-depth look at its chemical properties:
Tin primarily exhibits two oxidation states: +2 and +4. The +2 state (stannous) is more reactive, while the +4 state (stannic) is more stable in organotin compounds.
Tin reacts with oxygen to form tin(IV) oxide when heated: Sn+O₂→SnO2
This oxide layer protects underlying tin from further oxidation.
Tin is resistant to corrosion and does not react with water, making it suitable for food containers and other protective coatings.
Tin reacts with strong acids to form tin(II) chloride and hydrogen gas: Sn+2HCl→SnCl₂+H₂
With oxidizing acids like nitric acid, tin can form tin(IV) oxide: 3Sn+4HNO3→3SnO₂+4NO₂+2H₂O
Tin easily forms alloys, such as bronze (with copper) and solder (with lead), altering the properties of the base metal for specific applications.
Organotin compounds are formed with tin bonded to organic groups. Tin(IV) chloride is a starting material for creating these compounds, used in various industrial applications: Sn+4RCl→R₄Sn+4HCl where R represents an organic group
Property | Value |
|---|---|
Melting Point | 231.93°C (449.47°F) |
Boiling Point | 2602°C (4716°F) |
Heat of Fusion | 7.03 kJ/mol |
Heat of Vaporization | 296.1 kJ/mol |
Specific Heat Capacity | 27.112 J/(mol·K) |
Thermal Conductivity | 66.8 W/(m·K) |
Property | Value |
|---|---|
Density | 7.31 g/cm³ |
Young’s Modulus | 50 GPa |
Tensile Strength | 14-200 MPa |
Hardness | 1.5 Mohs |
Malleability | High |
Ductility | High |
Property | Value |
|---|---|
Electrical Conductivity | 8.69 × 10⁶ S/m |
Magnetic Susceptibility | -3.8 × 10⁻⁵ |
Property | Description |
|---|---|
Atomic Number | 50 – Tin has 50 protons in its nucleus, defining its chemical properties. |
Atomic Mass | 118.71 u – The average mass of the tin atom, reflecting the sum of its protons and neutrons. |
Isotopes | Stable: 10 – Tin has the largest number of stable isotopes among all elements, contributing to its diverse applications. |
Neutron Cross Section | 0.626 barns – A measure of the probability of neutron capture, relevant in nuclear reactions and applications. |
Half-life of Most Stable Isotope | ^{126}Sn, 2.3 × 10⁵ years – Longest-lived radioactive tin isotope, though most natural tin is stable. |
Tin, symbolized as Sn (from the Latin ‘stannum’), is commonly extracted from its primary ore, cassiterite (SnO₂), through a smelting process that involves several key steps to produce pure tin metal. The preparation of tin can be summarized as follows:
Mining and Concentration: Cassiterite ore is mined from tin deposits and then concentrated through washing and gravity separation techniques to increase the SnO₂ content.
Roasting: The concentrated ore is then roasted in the presence of air to remove impurities such as sulfur, arsenic, and antimony as oxides. This process results in nearly pure SnO₂.
Reduction Smelting: The purified SnO₂ is mixed with a reducing agent, typically carbon (in the form of coal or coke), and heated in a furnace.
The chemical reaction that takes place is: SnO₂+2C→Sn+2CO
Purification: The crude tin obtained from smelting contains impurities like iron, copper, and lead. It is purified by heating on a sloping surface, where the higher-melting-point impurities remain solid while the molten tin flows away and is collected.
Electrolytic Refining: For further purification, electrolytic refining is used, where impure tin serves as the anode, and a thin sheet of pure tin acts as the cathode in an electrolyte solution. Upon applying a current, tin ions move to the cathode and deposit as pure tin.
This multi-step process ensures that the tin produced is of high purity, suitable for various industrial and commercial applications.
Tin, a versatile metal, forms numerous compounds exhibiting diverse chemical properties. Here are six significant chemical compounds of tin, along with their descriptions and relevant chemical equations:
Tin(II) Chloride (SnCl₂)
Description: Tin(II) Chloride is a white crystalline solid, soluble in water, and serves as a strong reducing agent. It’s used in electroplating, as a mordant in dyeing, and in the manufacturing of perfumes.
Equation:
Sn+2HCl→SnCl₂+H₂
Tin reacts with hydrochloric acid to form tin(II) chloride and hydrogen gas.
Tin(IV) Chloride (SnCl₄)
Description: Tin(IV) Chloride, also known as stannic chloride, is a colorless, fuming liquid with a pungent odor, used in organic syntheses and as a tin source for various chemical reactions.
Equation:
Sn+4HCl+O₂→SnCl₄+2H₂O
Tin reacts with chlorine in the presence of oxygen to produce tin(IV) chloride and water.
Tin(II) Oxide (SnO)
Description: Tin(II) Oxide is a black or blue-black powder, used in the making of glass and ceramic glazes. It acts as a reducing agent in organic synthesis.
Equation:
2Sn+O₂→2SnO
Tin reacts with oxygen to form tin(II) oxide.
Tin(IV) Oxide (SnO₂)
Description: Tin(IV) Oxide, or stannic oxide, is a white powdery substance used as a pigment, in putty, and for polishing glass. It’s the most stable oxide of tin.
Equation:
Sn+O₂→SnO₂
Tin reacts with oxygen to produce tin(IV) oxide.
Tin(II) Sulfide (SnS)
Description: Tin(II) Sulfide is a dark brown or black solid, used in photocells and as a colorant in glazes. It’s one of the components of bronze patina.
Equation:
Sn+S→SnS
Tin reacts with sulfur to form tin(II) sulfide.
Tin(IV) Sulfide (SnS₂)
Description: Tin(IV) Sulfide, also known as mosaic gold, is a yellow solid used for bronzing and in decorative coatings. It’s applied in the manufacture of certain types of solar cells.
Equation:
Sn+2S→SnS₂
Tin reacts with sulfur to produce tin(IV) sulfide
Tin is unique among all elements for having the largest number of stable isotopes, with ten recognized by the scientific community. These isotopes range from tin-112 to tin-124, differing in their number of neutrons. Here’s a brief overview of tin’s isotopes:
Stable Isotopes: Tin-112, Tin-114, Tin-115, Tin-116, Tin-117, Tin-118, Tin-119, Tin-120, Tin-122, and Tin-124. These isotopes contribute to tin’s average atomic mass and have various natural abundances.
Radioactive Isotopes: In addition to its stable isotopes, tin has over 30 radioactive isotopes, with Tin-126 being the most stable among them. These isotopes are primarily of interest in scientific research and have shorter half-lives compared to the stable isotopes.
The diversity of tin’s isotopes is valuable for research in nuclear physics and isotopic geochemistry, providing insights into processes ranging from the formation of solar systems to the behavior of materials under extreme conditions
Tin’s physical and chemical properties make it an essential material in various applications across multiple industries. Here are some of the primary uses of tin:
Solder: Tin is a major component in solder, an alloy used widely in the electronics industry for joining components.
Plating and Coatings: Tin plating is used to coat other metals to prevent corrosion and rust, particularly in food packaging, such as tin cans.
Alloys: Tin forms important alloys such as bronze (with copper) and pewter (with antimony and copper), which are used in coins, sculptures, and decorative items.
Chemicals: Organotin compounds are used as stabilizers in PVC plastics, as catalysts in the production of polyurethane foams and silicone elastomers, and in the manufacture of glass coatings.
Glass Production: Tin(IV) oxide is used as a polishing agent for glass and ceramics and to produce opaque glass.
Batteries: Tin-based materials are explored as potential anodes in lithium-ion and other types of batteries due to their high energy density
The commercial production of tin involves several steps, from mining tin ore to refining and processing it into usable forms. The primary source of tin is cassiterite (tin oxide, SnO2), from which tin is extracted through the following processes:
Mining: Tin is mined from both primary deposits (where tin is found in ores) and secondary deposits (where tin is recovered from recycling).
Concentration: The ore is crushed and ground to liberate tin oxide from the rest of the material, followed by a concentration process, usually involving gravity separation or flotation, to increase the tin content.
Smelting: The concentrated ore is then smelted in a furnace where it is heated with a carbon source (like coal or coke) to reduce the tin oxide to metallic tin.
Refining: The crude tin obtained from smelting is refined to remove impurities. This can involve electrolytic refining or vacuum distillation, resulting in high-purity tin ready for commercial use
Tin, in its metallic form, is relatively non-toxic and poses little risk to human health. However, certain tin compounds and organotin compounds can have harmful health effects:
Organotin Compounds: These are more toxic, particularly triorganotins, which can affect the central nervous system, skin, liver, and immune system. They can also be endocrine disruptors.
Inhalation: Inhaling tin dust or fumes during smelting or refining processes can cause respiratory problems.
Ingestion: Consuming food or drink contaminated with certain tin compounds can lead to stomachaches, liver and kidney problems, and in severe cases, neurological damage.
Proper handling and industrial hygiene practices are essential to minimize exposure and prevent health risks associated with tin compounds.
Tin’s environmental impact is primarily associated with mining and processing operations. These effects can include:
Habitat Destruction: Mining activities can lead to deforestation and the destruction of natural habitats, affecting local biodiversity.
Soil and Water Contamination: The release of tin and other chemicals used in ore processing can contaminate soil and water sources, posing risks to wildlife and potentially entering the human food chain.
Resource Depletion: The extraction of non-renewable resources like tin contributes to the depletion of natural resources.
Tin is called “Sn” because the symbol derives from the Latin name for tin, “stannum.” This nomenclature is part of the international system of chemical symbols used to represent elements based on their Latin names, facilitating universal understanding and communication in the scientific community
Tin is used in a wide array of applications, including soldering materials for electronics, coatings for steel cans to prevent corrosion, glass-making, and creating various alloys like bronze. Its versatility stems from its corrosion resistance, malleability, and low melting point, making it invaluable in industrial and consumer products
Tin is a pure metal in its elemental form, classified under the category of metals in the periodic table. It exists naturally in the Earth’s crust, primarily obtained from the mineral cassiterite (SnO2), and can be processed into a highly pure state for various commercial and industrial applications
In the periodic table, tin is represented by the symbol “Sn” and has an atomic number of 50. It is situated in group 14, also known as the carbon group. This positioning reflects its chemical properties, including the ability to form compounds in multiple oxidation states and its role as a post-transition metal
Tin is a versatile metal with widespread applications in electronics, coatings, and alloys, owing to its distinct physical and chemical properties. While its commercial production is crucial for various industries, attention to the health and environmental impacts of tin and its compounds is essential. Responsible management and recycling can mitigate these effects, sustaining its valuable role in technological advancements and daily use.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the primary use of tin in modern industries?
Wiring
Solder
Building construction
Pharmaceuticals
Which alloy of tin is commonly used for coating to prevent corrosion?
Bronze
Brass
Pewter
Tinplate
What environmental issue is associated with tin mining?
Deforestation
Air pollution
Water pollution
Soil erosion
In which historical period was tin first used?
Iron Age
Bronze Age
Neolithic
Medieval
What property of tin makes it useful in tin cry?
Elasticity
Brittleness at low temperatures
High melting point
Malleability
What is a common application of tin in the food industry?
Flavor enhancer
Coloring agent
Preservative
Can coating
How is tin most commonly extracted?
Leaching
Smelting
Electrolysis
Distillation
Which of the following is a characteristic feature of organotin compounds?
Solubility in water
Stability in high temperatures
Biodegradability
Use as biocides
What impact does tin have when released into freshwater ecosystems?
Increases algae growth
Harms aquatic plants
Disrupts fish reproduction
None of the above
What global organization regulates the trade and use of tin?
United Nations
World Trade Organization
International Tin Association
Global Mining Consortium
Before you leave, take our quick quiz to enhance your learning!

