What is the atomic number of Uranium?
90
991
92
93
Uranium, a cornerstone of the nuclear energy sector, embodies a blend of power and complexity. This guide unveils the essence of uranium, exploring its multifaceted roles from fueling atomic reactors to its critical applications in medicine and industry. With a focus on safe extraction, handling, and innovative uses, we dive deep into the science and technology behind uranium’s global influence. Discover the transformative impact of this mighty element, underscoring its significance in advancing sustainable energy solutions and pushing the boundaries of scientific research
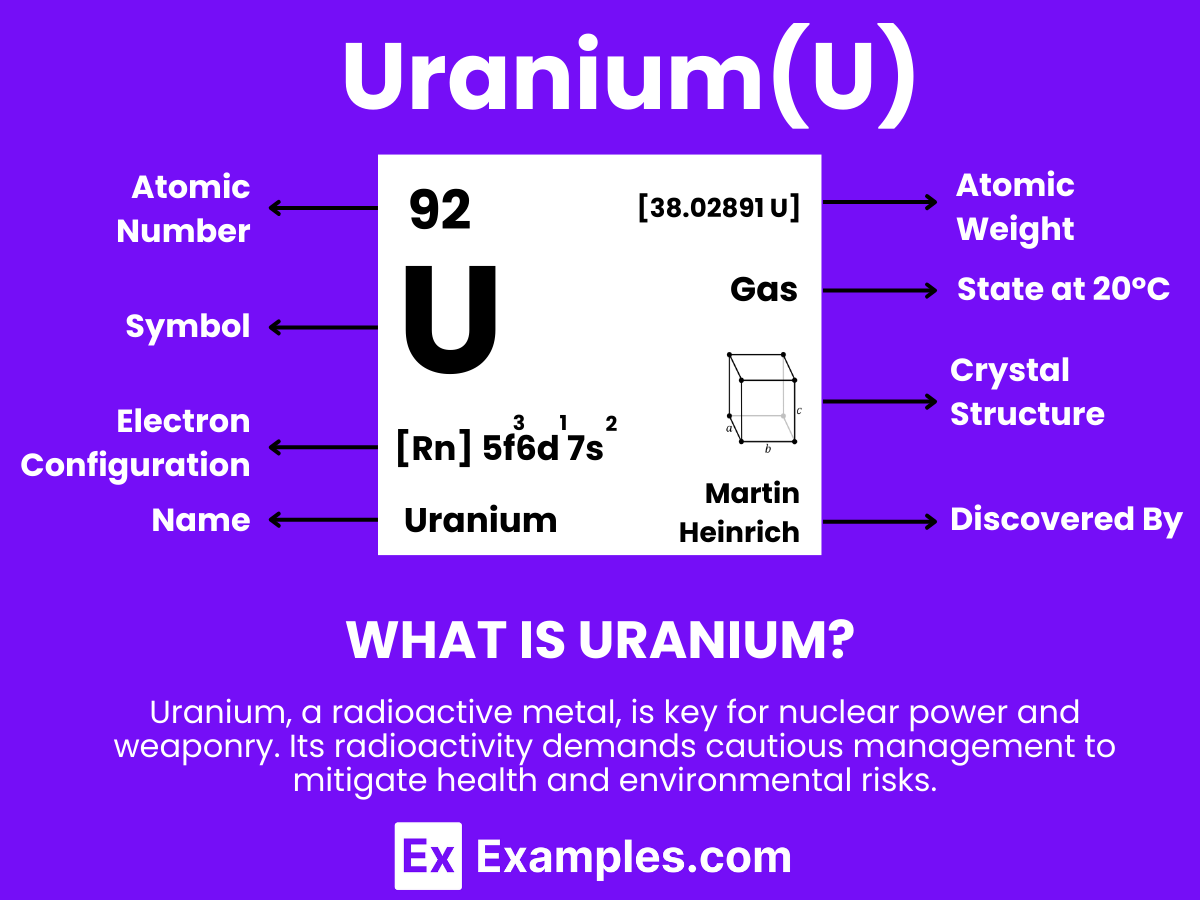
Uranium is a heavy, naturally occurring, radioactive metal with the chemical symbol U and atomic number 92. It stands out for its dense properties and is well-known for its significant role in nuclear energy and weaponry. This element is found in small amounts in rocks, soil, and water and is mined from the Earth’s crust. Uranium is notable for its ability to undergo nuclear fission, a process where its atoms split to release a large amount of energy, which is harnessed in nuclear reactors and atomic bombs. Despite its radioactivity, uranium also has uses in medicine, space exploration, and as a colorant in glass and ceramics.
Uranium, in stark contrast to hydrogen and even gallium, is a heavy metal known for its significant role in nuclear chemistry rather than its occurrence in gaseous form. Its physical and chemical properties are markedly influenced by its position in the periodic table and its actinide nature.
Atomic Level: Each uranium atom (U) contains 92 protons in its nucleus and has 92 electrons orbiting around it. The electron configuration of uranium is [Rn] 5f³ 6d¹ 7s², which highlights it has three electrons in its 5f orbital and one in its 6d orbital available for bonding.
Molecular Formation: Uranium, like gallium, does not form diatomic molecules as hydrogen does (H₂). In its solid state, uranium atoms are arranged in a complex crystalline structure. This arrangement involves metallic bonding, where electrons are shared among a lattice of uranium atoms. Upon melting, uranium transitions to a liquid while maintaining metallic bonding characteristics, which accounts for its high density and surface tension in the liquid state.
Uranium’s lattice bonds are robust, enabling it to preserve its solid state up to its melting point of about 1132.2°C (2070°F). Contrary to hydrogen, which is gaseous at room temperature, uranium remains solid under standard conditions and melts at temperatures significantly higher than room temperature. Given its very high boiling point of approximately 4131°C (7468°F), uranium does not naturally exist as a gas or in a diatomic gaseous state under normal conditions. The concept of “Uranium Gas” primarily relates to uranium hexafluoride (UF6), a compound used in the gas phase for uranium enrichment processes, rather than elemental uranium in a gaseous state.
| Property | Description |
|---|---|
| Appearance | Silvery-gray metallic |
| Atomic Number | 92 |
| Density (at 20°C) | 19.1 g/cm³ |
| Melting Point | 1132.2°C (2070°F) |
| Boiling Point | 4131°C (7468°F) |
| State at Room Temperature | Solid |
| Electron Configuration | [Rn]5f36d17s2 |
| Common Oxidation States | +3, +4, +6 |
Uranium exhibits several chemical properties that are significant both in nature and in various applications, particularly in nuclear science.
| Property | Value with Unit |
|---|---|
| Boiling Point | 4131 °C |
| Melting Point | 1132.2 °C |
| Critical Temperature | Not Available |
| Critical Pressure | Not Available |
| Heat of Vaporization | 417.1 kJ/mol |
| Heat of Fusion | 9.14 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.116 J/g·K |
| Thermal Conductivity | 27.5 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 20°C) | 19.1 g/cm³ |
| Viscosity | Not Applicable (Solid) |
| Solubility in Water | Insoluble |
| Color | Metallic silver to dull gray |
| Phase at Room Temperature | Solid |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 0°C) | 0.28 µΩ·m |
| Thermal Conductivity | 27.5 W/m·K |
| Magnetic Susceptibility | +40.9 × 10^-6 cm^3/mol |
| Electronegativity (Pauling scale) | 1.38 |
| Property | Value with Unit |
|---|---|
| Atomic Number | 92 |
| Atomic Mass | 238.02891 u |
| Isotopes | ^238U (99.2745%), ^235U (0.720%), ^234U (0.0055%) |
| Half-Life (for ^238U) | 4.468 × 10^9 years |
| Half-Life (for ^235U) | 7.04 × 10^8 years |
| Half-Life (for ^234U) | 245,500 years |
| Nuclear Spin (for ^235U) | 7/2 ℏ |
| Neutron Cross Section (for ^238U) | 2.68 barns |
| Neutron Cross Section (for ^235U) | 681 barns (thermal neutron) |
The preparation of uranium from its ores involves several complex processes that extract and purify uranium. This multi-step process is essential for producing uranium suitable for use in nuclear reactors and weapons.
Spent nuclear fuel and other uranium-containing wastes are handled with care due to their radioactivity and potential environmental impact. Reprocessing spent fuel can recover unused uranium and plutonium, which can be reused as fuel, although this process is complex and involves significant safety and non-proliferation considerations.
The preparation of uranium is a highly technical and regulated process, reflecting its importance in energy production and national security, as well as the need for careful management of radioactive materials.
Uranium has several isotopes, each with unique properties and applications. The most significant isotopes of uranium include:
Uranium’s uses are diverse, ranging from energy production to military applications and scientific research:
The production of uranium involves several key steps to extract, process, and refine the raw uranium ore into a usable form for energy production, military applications, and other uses. Here’s an overview of the uranium production process:
Uranium’s primary applications are centered around its energy-producing capabilities and its density. Here are some of the key uses:
In summary, uranium plays a pivotal role in modern society, primarily fueling nuclear energy production and national defense through its use in nuclear weapons. Its unique properties, from high density to radioactive nature, also find applications in medicine, industry, and science. The careful extraction, processing, and management of uranium underscore its significance and the need for responsible stewardship.

Uranium, a cornerstone of the nuclear energy sector, embodies a blend of power and complexity. This guide unveils the essence of uranium, exploring its multifaceted roles from fueling atomic reactors to its critical applications in medicine and industry. With a focus on safe extraction, handling, and innovative uses, we dive deep into the science and technology behind uranium’s global influence. Discover the transformative impact of this mighty element, underscoring its significance in advancing sustainable energy solutions and pushing the boundaries of scientific research
Uranium is a heavy, naturally occurring, radioactive metal with the chemical symbol U and atomic number 92. It stands out for its dense properties and is well-known for its significant role in nuclear energy and weaponry. This element is found in small amounts in rocks, soil, and water and is mined from the Earth’s crust. Uranium is notable for its ability to undergo nuclear fission, a process where its atoms split to release a large amount of energy, which is harnessed in nuclear reactors and atomic bombs. Despite its radioactivity, uranium also has uses in medicine, space exploration, and as a colorant in glass and ceramics.
Formula: U
Composition: Consists of a single uranium atom.
Bond Type: In its elemental form, uranium does not have bonds as it is a pure element. However, uranium can form covalent or ionic bonds when reacting with other elements.
Molecular Structure: As a pure element, uranium does not form a molecular structure in the same way as compounds like H₂. At room temperature, uranium is in a metallic state with a complex crystalline structure, often orthorhombic or tetragonal.
Electron Sharing: In compounds, uranium typically shares electrons covalently or transfers electrons ionically, depending on the nature of the other element(s) it is bonding with.
Significance: Uranium is notable for its high density and radioactivity. It is the principal fuel for nuclear reactors and the main material for nuclear weapons. Its ability to undergo fission makes it a critical energy source.
Role in Chemistry: Uranium plays a vital role in nuclear chemistry and energy production. It forms a variety of compounds, including uranium dioxide (UO₂) and uranium hexafluoride (UF₆), which are essential in the nuclear fuel cycle and in the enrichment process, marking it as a key material in nuclear technology applications.
Uranium, in stark contrast to hydrogen and even gallium, is a heavy metal known for its significant role in nuclear chemistry rather than its occurrence in gaseous form. Its physical and chemical properties are markedly influenced by its position in the periodic table and its actinide nature.
Atomic Level: Each uranium atom (U) contains 92 protons in its nucleus and has 92 electrons orbiting around it. The electron configuration of uranium is [Rn] 5f³ 6d¹ 7s², which highlights it has three electrons in its 5f orbital and one in its 6d orbital available for bonding.
Molecular Formation: Uranium, like gallium, does not form diatomic molecules as hydrogen does (H₂). In its solid state, uranium atoms are arranged in a complex crystalline structure. This arrangement involves metallic bonding, where electrons are shared among a lattice of uranium atoms. Upon melting, uranium transitions to a liquid while maintaining metallic bonding characteristics, which accounts for its high density and surface tension in the liquid state.
Uranium’s lattice bonds are robust, enabling it to preserve its solid state up to its melting point of about 1132.2°C (2070°F). Contrary to hydrogen, which is gaseous at room temperature, uranium remains solid under standard conditions and melts at temperatures significantly higher than room temperature. Given its very high boiling point of approximately 4131°C (7468°F), uranium does not naturally exist as a gas or in a diatomic gaseous state under normal conditions. The concept of “Uranium Gas” primarily relates to uranium hexafluoride (UF6), a compound used in the gas phase for uranium enrichment processes, rather than elemental uranium in a gaseous state.
Property | Description |
|---|---|
Appearance | Silvery-gray metallic |
Atomic Number | 92 |
Density (at 20°C) | 19.1 g/cm³ |
Melting Point | 1132.2°C (2070°F) |
Boiling Point | 4131°C (7468°F) |
State at Room Temperature | Solid |
Electron Configuration | [Rn]5f36d17s2 |
Common Oxidation States | +3, +4, +6 |
Uranium exhibits several chemical properties that are significant both in nature and in various applications, particularly in nuclear science.
Reactivity with Air: Uranium reacts with air to form a protective oxide layer, preventing further oxidation. This layer can be represented by the formation of uranium dioxide (UO₂) or triuranium octoxide (U₃O₈).
U+3O₂→2U₂O₃
U+4O₂→U₃O₈
Reactivity with Water: In water, uranium reacts slowly with cold water and more rapidly with hot water, forming uranium dioxide and releasing hydrogen gas.
U+2H₂O→UO₂+2H₂
Reactivity with Acids: Uranium dissolves in hydrochloric acid and sulfuric acid to form uranium chloride (UCl₄) and uranium sulfate (U(SO₄)₂), respectively, along with hydrogen gas.
U+4HCl→UCl₄+2H₂
U+H₂SO4→U(SO4)₂+H₂
Formation of Uranium Hexafluoride (UF₆): Uranium reacts with fluorine gas at 500°C to form uranium hexafluoride, a compound used in the gas centrifuge process for uranium enrichment.
U+3F₂→UF₆
Radioactivity: Uranium is naturally radioactive, primarily emitting alpha particles, which plays a critical role in its use as fuel for nuclear reactors and in the production of nuclear weapons.
Ability to Undergo Fission: Uranium-235, one of the isotopes of uranium, is capable of undergoing induced fission, a process in which the nucleus of an atom splits into two smaller nuclei along with the release of energy.
²³⁵U+n→ Fission Products +2-3 n+Energy
Formation of Complex Compounds: Uranium forms a variety of complex compounds with other elements, including carbonates and nitrates, indicating its versatile coordination chemistry and reactivity.
Example: UO2(CO₃)₃⁴− for a complex with carbonate ions.
Property | Value with Unit |
|---|---|
Boiling Point | 4131 °C |
Melting Point | 1132.2 °C |
Critical Temperature | Not Available |
Critical Pressure | Not Available |
Heat of Vaporization | 417.1 kJ/mol |
Heat of Fusion | 9.14 kJ/mol |
Specific Heat Capacity (at 25°C) | 0.116 J/g·K |
Thermal Conductivity | 27.5 W/m·K |
Property | Value with Unit |
|---|---|
Density (at 20°C) | 19.1 g/cm³ |
Viscosity | Not Applicable (Solid) |
Solubility in Water | Insoluble |
Color | Metallic silver to dull gray |
Phase at Room Temperature | Solid |
Property | Value with Unit |
|---|---|
Electrical Resistivity (at 0°C) | 0.28 µΩ·m |
Thermal Conductivity | 27.5 W/m·K |
Magnetic Susceptibility | +40.9 × 10^-6 cm^3/mol |
Electronegativity (Pauling scale) | 1.38 |
Property | Value with Unit |
|---|---|
Atomic Number | 92 |
Atomic Mass | 238.02891 u |
Isotopes | ^238U (99.2745%), ^235U (0.720%), ^234U (0.0055%) |
Half-Life (for ^238U) | 4.468 × 10^9 years |
Half-Life (for ^235U) | 7.04 × 10^8 years |
Half-Life (for ^234U) | 245,500 years |
Nuclear Spin (for ^235U) | 7/2 ℏ |
Neutron Cross Section (for ^238U) | 2.68 barns |
Neutron Cross Section (for ^235U) | 681 barns (thermal neutron) |
The preparation of uranium from its ores involves several complex processes that extract and purify uranium. This multi-step process is essential for producing uranium suitable for use in nuclear reactors and weapons.
Mining: Uranium is extracted from the earth through conventional mining techniques (open-pit or underground mining) or by in-situ leaching (ISL), where solutions are injected into underground deposits to dissolve the uranium.
Milling: The mined uranium ore is crushed and processed in a mill to extract uranium oxide concentrate, commonly known as yellowcake (U₃O₈). This process involves grinding the ore, treating it with acid or alkali to dissolve the uranium, and then precipitating uranium oxide.
The yellowcake is then converted into uranium hexafluoride (UF₆), a gas used in the enrichment process. This involves reacting uranium oxide with hydrofluoric acid to form uranium tetrafluoride (UF₄) and then further fluorinating it with fluorine gas.
U₃O₈+6HF→3UF₄+4H₂O
6UF₄+F₂→UF₆
Uranium found in nature consists mostly of uranium-238, with only about 0.7% being the fissionable isotope uranium-235. Enrichment increases the proportion of uranium-235. Methods include gas centrifuge and gaseous diffusion, both exploiting the slight mass difference between the uranium isotopes to separate them.
During enrichment, UF₆ gas is spun in centrifuges or forced through membranes to concentrate U-235.
After enrichment, the UF₆ is converted into uranium dioxide (UO₂) powder, which is then pressed into pellets. These pellets are baked in a high-temperature furnace to create a hard ceramic material.
UF₆→UO²
The UO₂ pellets are loaded into metal tubes to form fuel rods, which are then assembled into fuel assemblies for use in nuclear reactors.
Spent nuclear fuel and other uranium-containing wastes are handled with care due to their radioactivity and potential environmental impact. Reprocessing spent fuel can recover unused uranium and plutonium, which can be reused as fuel, although this process is complex and involves significant safety and non-proliferation considerations.
The preparation of uranium is a highly technical and regulated process, reflecting its importance in energy production and national security, as well as the need for careful management of radioactive materials.

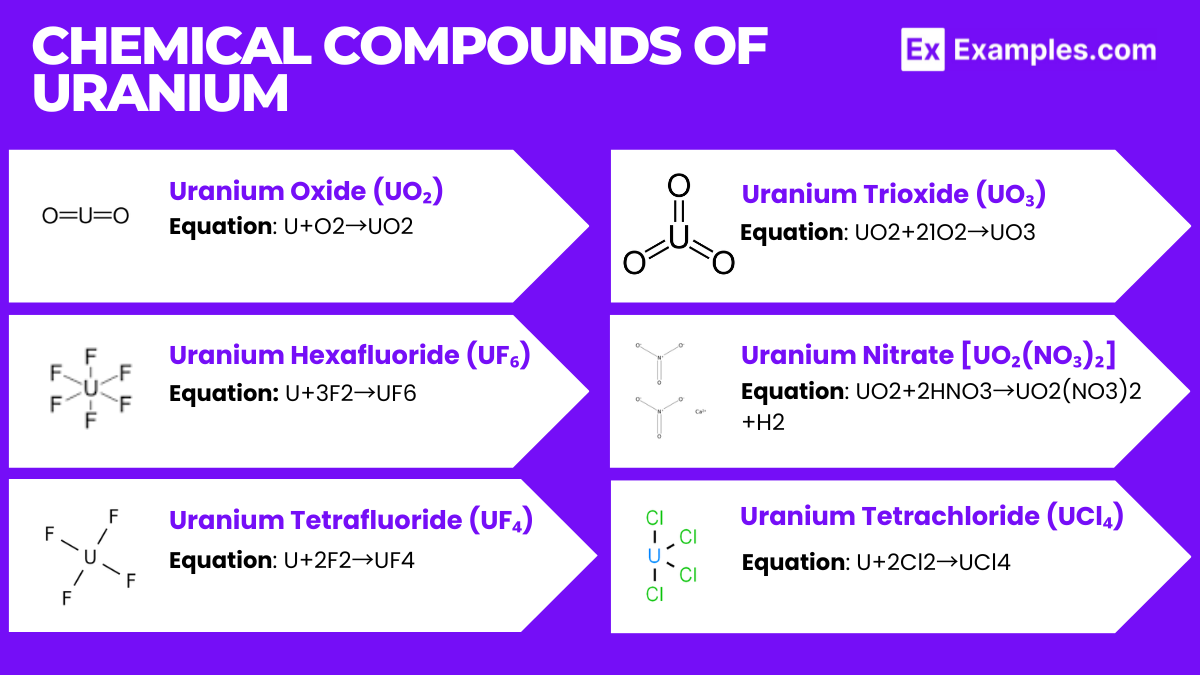
Description: Uranium dioxide or urania is a black, radioactive powder and is the most common form of uranium used as nuclear reactor fuel.
U+O₂→UO₂
Uses: It is the primary form used in uranium fuel rods for nuclear reactors.
Description: A white or light-colored compound that is solid at room temperature but sublimates at slightly above room temperature to a gas.
U+3F₂→UF₆
Uses: UF₆ is used in the gas centrifuge and gaseous diffusion processes for enriching uranium-235 from natural uranium.
Description: A green crystalline solid, known as “green salt.”
Formation Equation: U+2F₂→UF₄
Uses: It serves as an intermediate in the conversion of uranium dioxide to uranium hexafluoride and is also used in the uranium metal production process.
Description: An orange powder, less common than UO₂.
UO₂+21O₂→UO₃
Uses: It is used as an intermediate in the conversion of uranium ores to uranium metal or uranium hexafluoride.
Description: A soluble compound that forms yellow crystals.
Formation Equation: UO₂+2HNO₃→UO₂(NO₃)₂+H₂
Uses: Uranium nitrate is used in the reprocessing and enrichment of uranium and as a precursor for uranium fuel fabrication.
Description: A brown or purple crystalline solid.
Formation Equation: U+2Cl₂→UCl₄
Uses: It is used in the production of uranium metal and is also a starting material for other uranium compounds.
Description: An intermediate oxide that forms during the oxidation of UO₂ or the reduction of UO₃.
Formation Equation: 2UO₃+U→3UO₂
Uses: Less common, but it plays a role in various chemical processes involving uranium oxides.
Uranium has several isotopes, each with unique properties and applications. The most significant isotopes of uranium include:
Uranium-238 (U-238): The most abundant isotope of uranium, making up about 99.3% of natural uranium. It is not fissile (cannot sustain a nuclear chain reaction) but is fertile, meaning it can be converted into a fissile material (plutonium-239) in a nuclear reactor.
Uranium-235 (U-235): This isotope constitutes about 0.7% of natural uranium. It is fissile and is the primary isotope used as fuel in nuclear reactors and in the manufacturing of atomic bombs. Its ability to undergo fission makes it critically important for nuclear energy and weapons.
Uranium-234 (U-234): Found in trace amounts in natural uranium, this isotope is a product of U-238 decay. It has applications in dating geological formations and ocean waters.
Uranium’s uses are diverse, ranging from energy production to military applications and scientific research:
Nuclear Power: The primary use of uranium is as fuel for nuclear reactors. Enriched U-235 is used to generate electricity by undergoing fission, releasing a significant amount of heat, which is then used to produce steam for turbines.
Nuclear Weapons: Highly enriched U-235 is used in the manufacture of nuclear weapons due to its ability to undergo rapid, uncontrolled fission reactions.
Radioactive Dating: Uranium isotopes, particularly U-238, are used in radiometric dating to determine the age of rocks, minerals, and Earth itself.
Medical Applications: Radioactive isotopes of uranium are used in certain medical treatments and diagnostics. For example, small amounts of uranium can be used in medical imaging to analyze kidney function.
Research: Uranium isotopes play a role in research, including studies on nuclear physics, material science, and environmental science.
Counterweights and Shields: Due to its high density, depleted uranium (mostly U-238 after most U-235 has been extracted) is used in counterweights for aircraft, radiation shielding, and armor-piercing ammunition.
The production of uranium involves several key steps to extract, process, and refine the raw uranium ore into a usable form for energy production, military applications, and other uses. Here’s an overview of the uranium production process:
Mining: Uranium is extracted from the ground using one of three methods: open-pit mining, underground mining, or in-situ leach (ISL) mining. The choice of method depends on the depth and characteristics of the uranium deposit.
Milling: After mining, the ore is crushed and ground to liberate the uranium minerals from the rock. The ground ore is then treated with acids or alkalis to dissolve the uranium, producing a solution from which uranium can be precipitated.
Purification and Conversion: The precipitated uranium is then purified through a series of chemical processes. The purified uranium oxide concentrate, commonly referred to as “yellowcake” (U₃O₈), is converted into uranium hexafluoride (UF₆) gas in preparation for enrichment.
Enrichment: Natural uranium contains about 0.7% of the fissile isotope U-235. Enrichment processes, such as gas centrifugation or gaseous diffusion, increase the concentration of U-235 to levels suitable for use in nuclear reactors or weapons.
Fuel Fabrication: Enriched uranium hexafluoride is converted into uranium dioxide (UO₂) powder, which is then pressed into fuel pellets. These pellets are loaded into fuel rods, which are assembled into fuel assemblies for use in nuclear reactors.
Uranium’s primary applications are centered around its energy-producing capabilities and its density. Here are some of the key uses:
Nuclear Energy: The most well-known use of uranium is as fuel for nuclear reactors. Fissile isotopes, like U-235, undergo nuclear fission when struck by neutrons, releasing a tremendous amount of heat used to generate electricity.
Nuclear Weapons: Highly enriched U-235 is used in the cores of nuclear weapons due to its capability to sustain a rapid, uncontrolled chain reaction leading to a massive release of energy.
Radioisotope Production: Uranium-238 is used to produce plutonium-239 in breeder reactors, which is also used for energy and in nuclear weapons. Other isotopes produced from uranium are used for medical diagnostics and treatments, as well as in scientific research.
Counterweights and Ballast: Depleted uranium (uranium from which most of the U-235 has been removed) is used for counterweights in aircraft, radiation shielding in medical radiation therapy and industrial radiography equipment, and armor-piercing military projectiles due to its high density.
Colorants and Catalysis: Small amounts of uranium can be used as a colorant in ceramics and glass, and certain uranium compounds are used as catalysts in organic chemistry.
In summary, uranium plays a pivotal role in modern society, primarily fueling nuclear energy production and national defense through its use in nuclear weapons. Its unique properties, from high density to radioactive nature, also find applications in medicine, industry, and science. The careful extraction, processing, and management of uranium underscore its significance and the need for responsible stewardship.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons (92)
Neutrons (146)
Protons (92)
What is the atomic number of Uranium?
90
991
92
93
What is the chemical symbol for Uranium?
Ur
U
Un
Urn
Which isotope of Uranium is commonly used as fuel in nuclear reactors?
U-234
U-235
U-236
U-238
What is the most common oxidation state of Uranium in its compounds?
+2
+3
+4
+6
Uranium is primarily obtained from which mineral?
Hematite
Bauxite
Galena
Uraninite
What is the primary use of Uranium in the modern world?
Weaponry
Construction
Nuclear fuel
Electronics
What is the half-life of Uranium-238?
4.5 billion years
1 million years
100,000 years
700 million years
Which process is used to enrich Uranium for use in nuclear reactors?
Electrolysis
Fractional distillation
Gas centrifugation
Froth flotation
Which of the following is a characteristic of Uranium metal?
Brittle
Dense and heavy
Highly reactive with water
Transparent
What color does Uranium glass typically exhibit under UV light?
Blue
Red
Green
Yellow
Before you leave, take our quick quiz to enhance your learning!

