Urea (CH₄N₂O) – Definition, Structure, Preparation, Properties, Uses, Side Effects
Urea is a fascinating molecular compound that plays a crucial role in both nature and various industries. As a fundamental concept in chemistry, urea is known for being the main nitrogen-containing substance in the urine of mammals. It’s not just a waste product, though! Urea is widely used in fertilizers to help plants grow, in cosmetic products for skin care, and even in medical treatments. Its unique structure makes it versatile and essential in many chemical reactions, showcasing the beauty and complexity of chemistry in everyday life. Understanding urea is not only about diving into the world of molecules and compounds but also about seeing the connections between science and the world around us.
What is Urea?
Structure of Urea
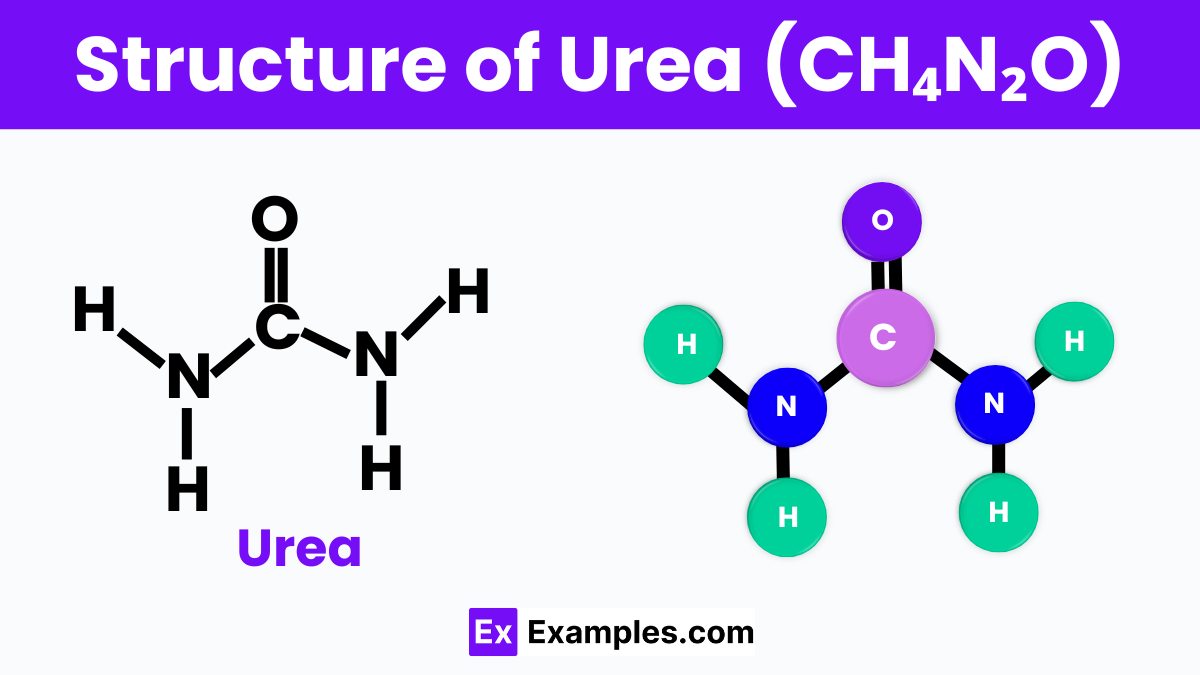
Urea has a neat and simple structure that makes it quite special. Imagine it as a little molecule with one carbon atom in the center, like the hub of a wheel. Attached to this carbon hub are two oxygen atoms and four hydrogen atoms, but they’re arranged in a specific way. Two of the hydrogen atoms team up with an oxygen atom to form something called an amine group, and these groups are like the spokes of the wheel, sticking out on opposite sides. The other oxygen atom is double-bonded directly to the carbon, creating a strong link. This setup helps urea do its job in our bodies and in the environment, acting like a carrier of nitrogen that plants love to use for growth. It’s like a tiny, efficient package, carrying important stuff for living things.
Preparation of Urea
Making urea is like cooking up a simple recipe in the lab! It starts with two main ingredients: ammonia (NH₃) and carbon dioxide (CO₂). When these gases are mixed together under high pressure and at a high temperature, they go through a special chemical reaction. This reaction forms urea (CO(NH₂)₂) and water (H₂O) as byproducts. Imagine it like mixing baking soda and vinegar, but under much stricter conditions, and instead of a fizz, you get urea!
In simple terms, for every two molecules of ammonia and one molecule of carbon dioxide you start with, you end up with one molecule of urea and one molecule of water.
Physical Properties of Urea
| Property | Description |
|---|---|
| Appearance | White, solid crystals or powder. |
| Solubility | Very soluble in water, meaning it dissolves easily. Also dissolves in ethanol and methanol. |
| Melting Point | About 133°C (271°F), which is the temperature it turns from solid to liquid. |
| Boiling Point | Decomposes before boiling, around 135°C (275°F), meaning it breaks down rather than boiling. |
| Odor | Odorless, making it comfortable to use since it doesn’t have any smell. |
| Taste | Slightly salty, but it’s not something to taste as it’s not food! |
| Density | 1.32 g/cm³, which tells us how compact it is. |
Chemical Properties of Urea
Hydrolysis
- Urea breaks down into ammonia and carbon dioxide when mixed with water, especially with heat or enzymes.
- Equation: CO(NH₂)₂ + H₂O → 2NH₃ + CO₂
Biuret Formation
- Heating urea above its melting point causes it to form biuret, a compound useful for identifying proteins.
- Equation: 2CO(NH₂)₂ → NH₂CONHCONH₂ + NH₃
Reactivity with Acids and Alkalis
Urea reacts with strong acids or bases to form new compounds, like urea nitrate with nitric acid.
Decomposition
- At temperatures over 180°C, urea decomposes into ammonia and isocyanic acid.
- Equation: CO(NH₂)₂ → NH₃ + HNCO
Uses of Urea

Fertilizer
Urea is a superstar in the gardening and farming world because it’s packed with nitrogen. Plants love nitrogen because it helps them grow big and strong. It’s like a superfood for crops!
Animal Feed
It’s also used in animal feed. Urea provides a source of nitrogen to animals, helping them make the protein they need to grow and stay healthy. It’s like a protein shake for livestock!
Industrial Uses
In industries, urea is a key ingredient in making plastics, adhesives, and even certain types of paper. It’s a bit like a building block that helps create all sorts of things we use every day.
Pharmaceutical Products
Urea is used in creams, ointments, and lotions for skin care. It helps moisturize the skin and can treat conditions like eczema. Think of it as a soothing balm for dry or irritated skin.
Pollution Reduction
Cars and trucks use a solution made from urea to reduce harmful emissions. This solution helps clean the exhaust gases, making the air cleaner for all of us. It’s like a bath for car exhaust.
Side Effects Of Urea
- Skin Irritation: For some people, using skin products with urea can cause itchiness, redness, or irritation, especially if the skin is sensitive.
- Allergic Reactions: Though rare, some individuals might experience allergic reactions to urea in skin care products, showing symptoms like rash, swelling, or intense itching.
- Over-fertilization: In agriculture, using too much urea as fertilizer can “burn” plants by overwhelming them with nitrogen, leading to yellowed, withered, or dead plants.
- Water Pollution: When used excessively in farming, urea can seep into groundwater, leading to water pollution. This can harm aquatic life and affect water quality.
FAQ’S
What is Urea in Human Body?
Urea is a waste product formed in the liver after protein breakdown, excreted through urine. It’s crucial for removing excess nitrogen from the body.
What Happens if Urea is Low?
Low urea levels might indicate a low protein diet, malnutrition, or liver problems, affecting the body’s ability to process waste effectively.
What Does it Mean if Urea is High?
High urea levels can signal kidney issues, dehydration, or excessive protein consumption, indicating the kidneys’ struggle to filter waste from the blood.
Urea (CH₄N₂O) – Definition, Structure, Preparation, Properties, Uses, Side Effects

Urea is a fascinating molecular compound that plays a crucial role in both nature and various industries. As a fundamental concept in chemistry, urea is known for being the main nitrogen-containing substance in the urine of mammals. It’s not just a waste product, though! Urea is widely used in fertilizers to help plants grow, in cosmetic products for skin care, and even in medical treatments. Its unique structure makes it versatile and essential in many chemical reactions, showcasing the beauty and complexity of chemistry in everyday life. Understanding urea is not only about diving into the world of molecules and compounds but also about seeing the connections between science and the world around us.
What is Urea?
Urea is a simple compound that plays a big role in our bodies and in farming. Think of it like a waste product that your body makes after it uses protein. Your liver breaks down the protein you eat, and urea is one of the leftovers. This substance is then sent to your kidneys, which get rid of it through your pee. But urea isn’t just about waste; it’s also a superstar in agriculture. Farmers use it as a fertilizer to help plants grow because it’s packed with nitrogen, a key nutrient that plants need to grow strong and healthy. So, urea is both a natural part of your body’s process and a helpful boost for crops in the field!
Structure of Urea
Urea has a neat and simple structure that makes it quite special. Imagine it as a little molecule with one carbon atom in the center, like the hub of a wheel. Attached to this carbon hub are two oxygen atoms and four hydrogen atoms, but they’re arranged in a specific way. Two of the hydrogen atoms team up with an oxygen atom to form something called an amine group, and these groups are like the spokes of the wheel, sticking out on opposite sides. The other oxygen atom is double-bonded directly to the carbon, creating a strong link. This setup helps urea do its job in our bodies and in the environment, acting like a carrier of nitrogen that plants love to use for growth. It’s like a tiny, efficient package, carrying important stuff for living things.
Preparation of Urea
Making urea is like cooking up a simple recipe in the lab! It starts with two main ingredients: ammonia (NH₃) and carbon dioxide (CO₂). When these gases are mixed together under high pressure and at a high temperature, they go through a special chemical reaction. This reaction forms urea (CO(NH₂)₂) and water (H₂O) as byproducts. Imagine it like mixing baking soda and vinegar, but under much stricter conditions, and instead of a fizz, you get urea!
2NH₃ + CO₂ → CO(NH₂)₂ + H₂O
In simple terms, for every two molecules of ammonia and one molecule of carbon dioxide you start with, you end up with one molecule of urea and one molecule of water.
Physical Properties of Urea
Property | Description |
|---|---|
Appearance | White, solid crystals or powder. |
Solubility | Very soluble in water, meaning it dissolves easily. Also dissolves in ethanol and methanol. |
Melting Point | About 133°C (271°F), which is the temperature it turns from solid to liquid. |
Boiling Point | Decomposes before boiling, around 135°C (275°F), meaning it breaks down rather than boiling. |
Odor | Odorless, making it comfortable to use since it doesn’t have any smell. |
Taste | Slightly salty, but it’s not something to taste as it’s not food! |
Density | 1.32 g/cm³, which tells us how compact it is. |
Chemical Properties of Urea
Hydrolysis
Urea breaks down into ammonia and carbon dioxide when mixed with water, especially with heat or enzymes.
Equation: CO(NH₂)₂ + H₂O → 2NH₃ + CO₂
Biuret Formation
Heating urea above its melting point causes it to form biuret, a compound useful for identifying proteins.
Equation: 2CO(NH₂)₂ → NH₂CONHCONH₂ + NH₃
Reactivity with Acids and Alkalis
Urea reacts with strong acids or bases to form new compounds, like urea nitrate with nitric acid.
Decomposition
At temperatures over 180°C, urea decomposes into ammonia and isocyanic acid.
Equation: CO(NH₂)₂ → NH₃ + HNCO
Uses of Urea
Fertilizer
Urea is a superstar in the gardening and farming world because it’s packed with nitrogen. Plants love nitrogen because it helps them grow big and strong. It’s like a superfood for crops!
Animal Feed
It’s also used in animal feed. Urea provides a source of nitrogen to animals, helping them make the protein they need to grow and stay healthy. It’s like a protein shake for livestock!
Industrial Uses
In industries, urea is a key ingredient in making plastics, adhesives, and even certain types of paper. It’s a bit like a building block that helps create all sorts of things we use every day.
Pharmaceutical Products
Urea is used in creams, ointments, and lotions for skin care. It helps moisturize the skin and can treat conditions like eczema. Think of it as a soothing balm for dry or irritated skin.
Pollution Reduction
Cars and trucks use a solution made from urea to reduce harmful emissions. This solution helps clean the exhaust gases, making the air cleaner for all of us. It’s like a bath for car exhaust.
Side Effects Of Urea
Skin Irritation: For some people, using skin products with urea can cause itchiness, redness, or irritation, especially if the skin is sensitive.
Allergic Reactions: Though rare, some individuals might experience allergic reactions to urea in skin care products, showing symptoms like rash, swelling, or intense itching.
Over-fertilization: In agriculture, using too much urea as fertilizer can “burn” plants by overwhelming them with nitrogen, leading to yellowed, withered, or dead plants.
Water Pollution: When used excessively in farming, urea can seep into groundwater, leading to water pollution. This can harm aquatic life and affect water quality.
FAQ’S
What is Urea in Human Body?
Urea is a waste product formed in the liver after protein breakdown, excreted through urine. It’s crucial for removing excess nitrogen from the body.
What Happens if Urea is Low?
Low urea levels might indicate a low protein diet, malnutrition, or liver problems, affecting the body’s ability to process waste effectively.
What Does it Mean if Urea is High?
High urea levels can signal kidney issues, dehydration, or excessive protein consumption, indicating the kidneys’ struggle to filter waste from the blood.


