What is water vapor?
Solid form of water
Gaseous form of water
Liquid form of water
Frozen form of water

Water vapor is the gaseous form of water, a crucial molecular compound in the study of chemistry. It’s like the invisible steam that rises from a hot cup of tea. Even though we can’t always see it, water vapor is everywhere in the air around us, especially when it’s warm or humid. It plays a crucial role in our planet’s weather and climate, helping to form clouds and rain. Water vapor is fascinating because it’s the same ingredient that makes up oceans, rivers, and ice, just floating freely in the air. This remarkable transformation from liquid to gas happens through a process called evaporation, where water heats up and escapes into the atmosphere, becoming part of the air we breathe.
The most common type, atmospheric water vapor, exists in the air around us. It’s crucial for weather patterns, climate regulation, and the water cycle, helping form clouds and precipitation. Atmospheric water vapor levels can greatly influence local and global climates.
Steam is water vapor produced by boiling water. It’s used extensively in industrial processes, cooking, and heating. Steam carries a significant amount of energy, making it ideal for tasks requiring high heat, like generating electricity in turbines.
This is steam heated beyond its boiling point without increasing pressure. Superheated steam is used in industrial processes where dry steam is required, as it doesn’t condense as quickly as regular steam, making it more efficient for drying and cleaning.
Saturation steam exists at a temperature where water and steam can coexist. It’s used in applications requiring a consistent temperature, such as in sterilization processes in healthcare and food industries, ensuring materials are not damaged by excessive heat.
Although not water vapor in the traditional sense, sublimation refers to the process where ice changes directly into vapor without becoming liquid first, like with dry ice (solid carbon dioxide). This principle is important in understanding phase changes and is used in refrigeration and preservation techniques.
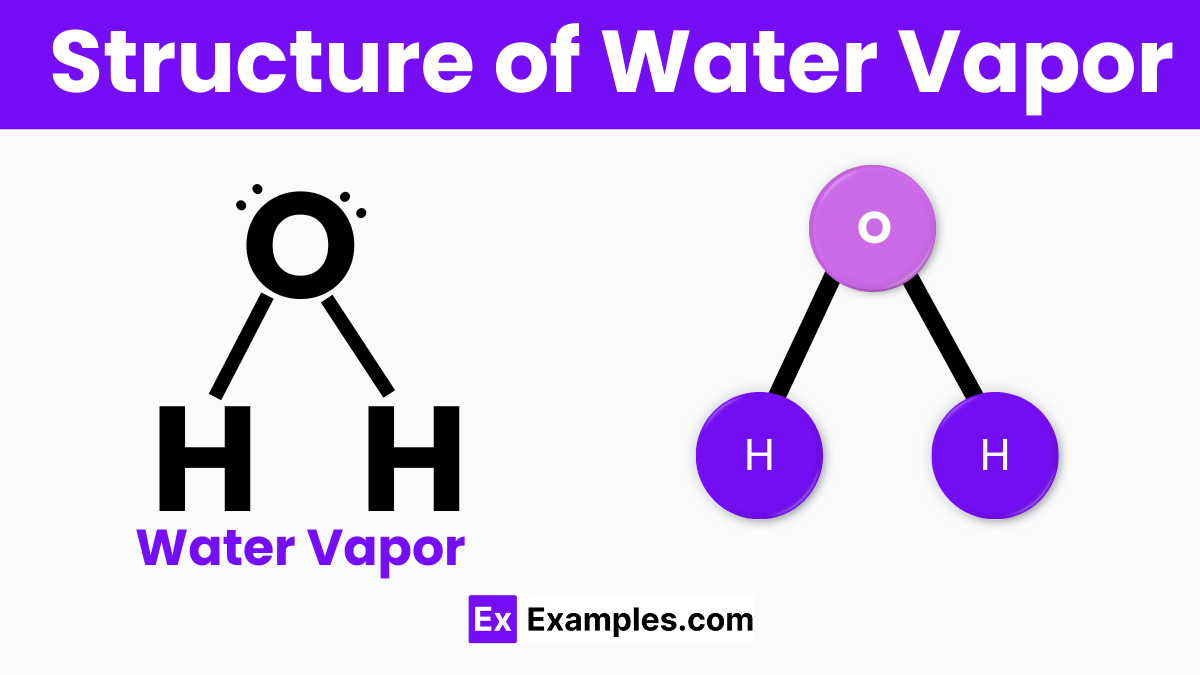
Water vapor, the gaseous form of water, has a simple yet fascinating structure that’s crucial to understanding its unique properties. Each water vapor molecule is made up of one oxygen atom bonded to two hydrogen atoms, forming a V-shape. This shape is due to the oxygen atom attracting more electrons, creating a slight negative charge near it, while the hydrogen atoms have a slight positive charge. This uneven distribution of electrical charge makes water molecules polar, meaning they have a positive end and a negative end.
This polarity allows water vapor molecules to form temporary bonds with each other, known as hydrogen bonds, even in their gaseous state. These bonds are weak and constantly breaking and reforming, giving water vapor its ability to absorb heat and play a significant role in Earth’s climate and the water cycle.
Preparing water vapor is a simple process that involves turning liquid water into its gas form through heating. When water is heated, it absorbs energy, causing the water molecules to move faster and spread out. Once the water reaches a temperature called the boiling point, at 212°F (100°C), it transforms into water vapor, a process known as boiling. This change from liquid to gas can be represented by the chemical equation:
The “(l)” signifies liquid water, and “(g)” indicates water in its gas form, or water vapor.
This process doesn’t change the chemical structure of water; it simply changes its physical state. In everyday life, boiling water on a stove is a common way to produce water vapor. Another way is through evaporation, where water slowly turns into vapor at temperatures below the boiling point, such as when clothes dry in the sun. Understanding how water vapor is prepared helps us grasp key concepts in chemistry and the natural water cycle that sustains life on Earth.
| Property | Description |
|---|---|
| State | Gas form of water, invisible to the naked eye. |
| Boiling Point | Converts to water vapor at 212°F (100°C) from liquid water. |
| Density | Lighter than air; its exact density depends on temperature and pressure. |
| Solubility | Can mix with many other gases, but its solubility decreases as the temperature increases. |
| Heat Capacity | High heat capacity, meaning it can absorb a lot of heat before its temperature rises significantly. |
| Conductivity | Poor conductor of electricity but can enhance the conductivity of air due to moisture content. |
Water vapor can react with certain metal oxides to form metal hydroxides. An example is its reaction with calcium oxide (lime), producing calcium hydroxide (slaked lime), crucial in construction. The reaction is represented by the equation: CaO(s) + H₂O(g) → Ca(OH)₂(s).
Water vapor possesses an amphiprotic nature, meaning it can act as both an acid and a base. This allows it to either donate a proton (H⁺), behaving as an acid, or accept a proton, acting as a base. This dual behavior is fundamental in various chemical reactions, including acid rain formation.
Though water vapor does not burn, it significantly influences combustion processes. Humidity levels can impact fuel combustion, often inhibiting it by absorbing heat. This property is vital for understanding how environmental conditions affect combustion engines.
The formation of water vapor occurs through the evaporation or boiling of liquid water (H₂O(l) → H₂O(g)), and it can return to liquid form upon cooling (H₂O(g) → H₂O(l)). These transformations are key components of the water cycle, allowing water to be distributed around the globe.
| Property | Value |
|---|---|
| CAS Registry Number | 7732-18-5 |
| PubChem Compound ID | 962 |
| PubChem Substance ID | 24851683 |
| SMILES Identifier | O |
| InChI Identifier | InChI=1/H2O/h1H2 |
| RTECS Number | ZC0110000 |
| MDL Number | MFCD00011332 |

Water vapor plays a critical role in weather patterns and climate regulation. It traps heat in the atmosphere, contributing to the greenhouse effect that warms the Earth, and forms clouds and precipitation, influencing weather.
In industries, water vapor is used in steam turbines to generate electricity. It’s also essential in the production of paper, textiles, and various chemicals, where steam helps in processes like drying, cleaning, and chemical reactions.
Steam cooking is a healthy way to prepare food, preserving nutrients and flavor. Water vapor heats food evenly and quickly, making it a preferred method in both home kitchens and professional culinary settings.
High-pressure steam is used to sterilize medical and laboratory equipment. This method effectively kills bacteria, viruses, and other pathogens, ensuring the safety of medical instruments and environments.
In dry climates or during winter, water vapor is added to the air in buildings through humidifiers to prevent dryness. This helps in maintaining a comfortable environment and in preventing respiratory issues.
Water vapor is crucial in greenhouses, where it’s used to regulate temperature and humidity. This creates an optimal growing environment for plants, enhancing their growth and health.
Water vapor acts as a natural thermostat, trapping heat in the atmosphere through the greenhouse effect. This helps regulate Earth’s temperature, making it habitable.
It plays a pivotal role in shaping weather patterns. Water vapor forms clouds and precipitation, influencing rain, snow, and storm occurrences globally.
Water vapor helps cleanse the environment. As it condenses, it can remove pollutants and dust from the air, contributing to cleaner, healthier air quality.
Plants rely on water vapor for transpiration, a process that supports their growth. It helps in nutrient absorption from the soil and regulates temperature within plant leaves.
In industries, water vapor is essential for power generation in steam turbines and in various manufacturing processes, including drying, cleaning, and chemical reactions.
Water vapor affects indoor air quality and comfort. Humidifiers add moisture to dry indoor air, improving skin hydration and respiratory health, especially in colder climates.
Yes, water vapor is still H2O. It’s simply water in its gaseous state, retaining its chemical identity but changing its physical form.
Air water vapor is the invisible gaseous form of water present in the atmosphere, essential for weather patterns and climate regulation.
Water vapor is also referred to as aqueous vapor, highlighting its nature as water in a gaseous state in various scientific contexts.
The term for vapor typically refers to the gaseous state of a substance that is liquid at room temperature, like water vapor.
The most common term used to describe water vapor is “humidity,” especially when referring to the amount of water vapor in the air.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is water vapor?
Solid form of water
Gaseous form of water
Liquid form of water
Frozen form of water
What process causes water to change into water vapor?
Melting
Evaporation
Freezing
Condensation
What happens when water vapor cools down?
It remains a gas
It turns into liquid water
It solidifies into ice
It forms crystals
In which part of the water cycle does water vapor form?
Precipitation
Evaporation
Runoff
Collection
Water vapor contributes to which weather phenomenon?
Tornadoes
Rain
Earthquakes
Volcanic eruptions
What happens to the water vapor in the air as humidity increases?
It decreases
It remains the same
It increases
It turns into ice
What role does water vapor play in the greenhouse effect?
It cools the Earth
It absorbs heat and traps it
It releases energy
It reflects sunlight
What is the term used to describe the amount of water vapor in the air?
Temperature
Pressure
Humidity
Density
What is the maximum amount of water vapor the air can hold called?
Relative humidity
Dew point
Absolute humidity
Saturation point
Which of the following processes directly reduces the amount of water vapor in the atmosphere?
Freezing
Evaporation
Condensation
Sublimation
Before you leave, take our quick quiz to enhance your learning!

