What type of bond holds the hydrogen and oxygen atoms together in a water molecule?
Ionic bond
Covalent bond
Metallic bond
Hydrogen bond

Water stands as a paramount covalent compound within the realm of chemistry. This molecule is composed of two hydrogen atoms bonded to a single oxygen atom through covalent bonds, a configuration that renders it essential for myriad biological processes and ecological systems. Its unique properties, such as its capability to dissolve a vast array of substances, high specific heat capacity, and surface tension, make water indispensable not only to life on Earth but also in numerous chemical reactions and industrial applications. As a covalent compound, water exemplifies the fundamental principles of chemical bonding, showcasing the intricate interplay of elements that defines the chemical world.
Water is symbolized as H₂O, is a simple yet vital substance made up of two hydrogen atoms bonded to one oxygen atom. It’s found everywhere on Earth, from vast oceans to tiny droplets in the air. This clear, tasteless, and odorless liquid is crucial for all forms of life, supporting plants, animals, and humans by helping in processes like drinking, cleaning, and growing food. Water also takes on different forms; it can be solid ice, liquid, or gas (steam), changing with the temperature. Its ability to dissolve many substances makes it an essential part of nature and daily life.
| Property | Value |
|---|---|
| Formula | H2O |
| Name | Water |
| Alternate Names | Dihydrogen Monoxide, Distilled Water, Hydrogen Hydroxide, Oxidane |
Water (H₂O) is a remarkable substance that exists in three primary states: liquid, solid, and gaseous. Each form exhibits unique characteristics and plays a crucial role in the Earth’s ecosystems and human activities.
Liquid water is the most familiar state to us, covering about 71% of the Earth’s surface as oceans, rivers, lakes, and rain. This form is indispensable for all known forms of life, serving as a medium for biological reactions, transportation of nutrients, and regulation of body temperature. Liquid water is unique for its high specific heat capacity, surface tension, and ability to dissolve more substances than any other liquid, making it known as the “universal solvent.”
When water freezes at 0°C (32°F), it becomes solid ice. Ice is less dense than liquid water, which is why it floats — a property crucial for aquatic life to survive under ice-covered waters in winter. Ice forms in various structures, from the vast ice sheets in Antarctica to the delicate frost patterns on a window. This solid state of water is also essential for activities such as ice skating and preserving food.
Water vapor, the gaseous form of water, is produced when water evaporates or boils. Steam is water in its gaseous state at temperatures above 100°C (212°F), where it becomes visible, like the steam from a boiling kettle. Water vapor, on the other hand, is invisible and a significant component of the Earth’s atmosphere, contributing to the greenhouse effect and the water cycle. This cycle, through evaporation and condensation, distributes heat and moisture around the globe, influencing weather patterns and climate.
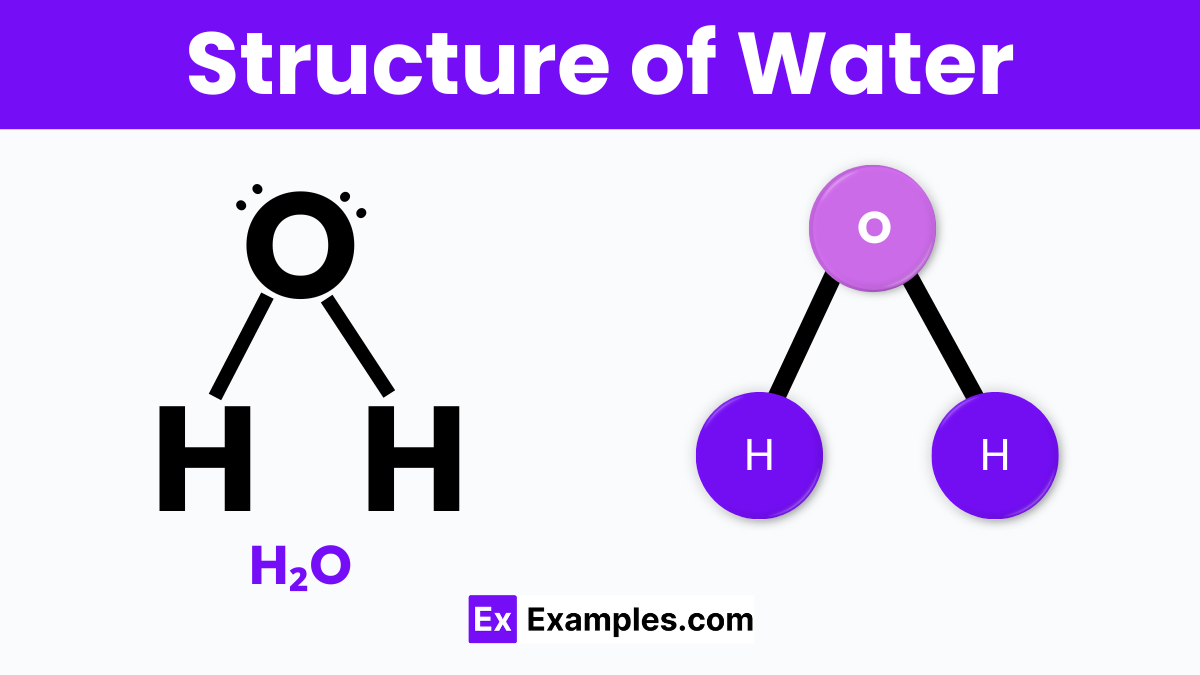
The structure of water (H₂O) is both simple and fascinating, making it one of the most important substances on Earth. At its core, a water molecule consists of two hydrogen atoms and one oxygen atom. These atoms are not just randomly attached; the two hydrogen atoms form a V-shape, or an angle, as they bond with the oxygen atom. This happens because the oxygen atom shares electrons with each hydrogen atom through a type of bond known as a covalent bond. This shared bonding gives water its liquid form at room temperature, unlike other similar molecules that might be gases.
What makes water really special is the way these molecules interact with each other. The side of the water molecule where the hydrogen atoms are is slightly positive, while the opposite side, near the oxygen, is slightly negative. This causes water molecules to be attracted to each other like tiny magnets, a property called hydrogen bonding. These bonds are strong enough to hold water molecules close together but flexible enough to let them move around freely, leading to water’s unique properties, such as its ability to dissolve many substances, its surface tension, and its unusual behavior when it freezes, expanding instead of contracting. This structure and the interactions it enables are crucial for life as we know it, affecting everything from the way our cells function to the weather patterns that shape our world.
The direct synthesis of water involves the reaction of hydrogen gas (H₂) with oxygen gas (O₂) to produce water. This process can be summarized by the chemical equation:
This reaction is highly exothermic, meaning it releases a significant amount of energy in the form of heat and light. In a controlled laboratory environment, this can be demonstrated by igniting a mixture of hydrogen and oxygen gases, where the resulting “explosion” produces water vapor. For safety and control, this reaction is usually carried out using a spark to ignite the gases in a controlled explosion chamber designed to contain the reaction’s energy.
Another method to prepare water, though seemingly counterintuitive, is the electrolysis of water itself. This process uses electrical energy to split water into its constituent elements, hydrogen and oxygen gas. The reverse process can then recombine these gases to form water, demonstrating both the decomposition and synthesis of water:
2H₂O(l)⟶2H₂(g)+O₂(g)
2H₂(g)+O₂(g)→2H₂O(l)
In this case, electrolysis serves as an educational tool, showing the reversible nature of chemical reactions and the conservation of matter.
| Property | Description |
|---|---|
| Molecular Weight | 18.01528 g/mol |
| State at Room Temperature | Liquid |
| Boiling Point | 100°C (212°F) at 1 atmosphere (atm) pressure |
| Freezing Point | 0°C (32°F) at 1 atm pressure |
| Density | 1 g/cm³ at 4°C (maximum density) |
| pH | 7 (neutral) at 25°C |
| Heat Capacity | High (about 4.186 joule/gram °C), meaning it can absorb a lot of heat before it gets hot. |
| Surface Tension | High (72.8 milliNewtons/m at 20°C), allowing it to form droplets and flow in narrow spaces. |
| Solvent Properties | Excellent solvent due to its polarity, capable of dissolving many substances. |
| Thermal Conductivity | 0.6065 W/(m·K) at 25°C, making it a good conductor of heat compared to other liquids. |
| Viscosity | 0.890 mPa·s at 25°C, indicating its resistance to flow. |
| Dielectric Constant | High (about 78.5 at 25°C), making it an effective medium for electrochemical reactions. |
Water molecules are polar, with the oxygen atom carrying a slight negative charge and the hydrogen atoms carrying slight positive charges. This polarity enables water to form hydrogen bonds with other molecules.
Water has a high specific heat capacity, allowing it to absorb or release large amounts of heat with minimal temperature change. The specific heat of water is approximately 4.186 J/g∘C.
Water ionizes slightly into hydronium and hydroxide ions:
H₂O(l)⇌H⁺(aq)+OH⁻(aq)
Water reaches its maximum density at 4°C. As it freezes, it expands:
H₂O(liquid)→H₂O(solid)
This expansion decreases its density, allowing ice to float on liquid water.
Water’s ability to dissolve many substances is due to its polarity, though no single equation defines this property. It’s the basis for many chemical reactions, such as salt dissolution:
NaCl(s)→Na⁺(aq)+Cl⁻(aq)
Water can be decomposed into oxygen and hydrogen gases by electrolysis, a process that requires the passage of an electric current, demonstrating its role in electrochemical reactions.
Pure water has a neutral pH of 7, indicating it’s neither acidic nor basic. The pH is determined by the concentration of hydronium ions:
H=−log[H3O⁺]
| Property | Value |
|---|---|
| CAS Registry Number | 7732-18-5 |
| PubChem Compound ID | 962 |
| PubChem Substance ID | 24851683 |
| SMILES Identifier | O |
| InChI Identifier | InChI=1/H2O/h1H2 |
| RTECS Number | ZC0110000 |
| MDL Number | MFCD00011332 |
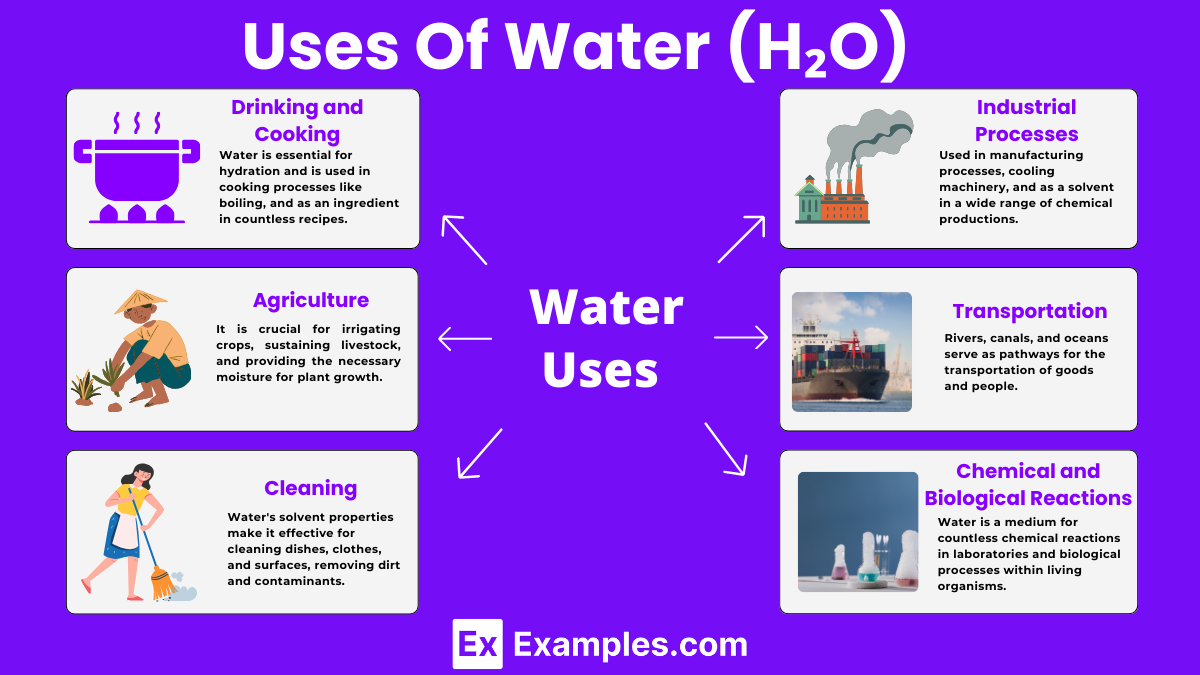
Water is essential for hydration and is used in cooking processes like boiling, steaming, and as an ingredient in countless recipes.
It is crucial for irrigating crops, sustaining livestock, and providing the necessary moisture for plant growth.
Water’s solvent properties make it effective for cleaning dishes, clothes, and surfaces, removing dirt and contaminants.
Used in manufacturing processes, cooling machinery, and as a solvent in a wide range of chemical productions.
Water is a key component in hydroelectric power plants, steam generation in thermal power plants, and cooling in nuclear reactors.
Rivers, canals, and oceans serve as pathways for the transportation of goods and people.
Water bodies are central to recreational activities like swimming, boating, and fishing.
Lakes, rivers, and oceans provide habitats for a diverse range of flora and fauna, supporting biodiversity.
Water is a medium for countless chemical reactions in laboratories and biological processes within living organisms.
The distribution and cycling of water influence weather patterns and help regulate the Earth’s temperature through processes like evaporation and precipitation.
Water is essential for maintaining body hydration, crucial for nearly all bodily functions, including regulation of body temperature, nutrient transport, and waste removal.
Water acts as a solvent, facilitating the transport and absorption of vitamins, minerals, and other nutrients in biological systems.
It helps in the elimination of waste products and toxins from the body through urine, sweat, and other excretory paths, supporting kidney function and overall detoxification.
Water aids in digestion by dissolving nutrients and helping to move food through the gut, preventing constipation and promoting healthy gastrointestinal function.
Adequate hydration keeps the skin moisturized, elastic, and healthy, reducing the risk of dry skin, wrinkles, and other skin issues.
Water has a high heat capacity, enabling it to play a critical role in regulating body temperature through sweating and respiration.
Proper hydration is linked to improved concentration, memory, and mood, as water comprises a significant portion of the brain.
Water lubricates and cushions joints, reducing the risk of joint pain and wear over time, important for movement and comfort.
Drinking water can aid in weight management by increasing satiety and enhancing metabolic rate, helping to control hunger and burn more calories.
Water supports ecosystems by providing habitats for diverse wildlife, facilitating nutrient cycles, and sustaining plant life, essential for biodiversity.
Water is vital for agriculture, enabling the growth of crops and sustaining livestock, which are foundational to food security.
Access to water boosts economic activities by supporting industries, agriculture, and energy production, crucial for development and prosperity.
Adults should aim for 8-10 cups (2-2.5 liters) of water daily, though needs vary by activity level, climate, and health.
Water hydrates, aids in nutrient transport, supports digestion, regulates temperature, and helps remove body wastes efficiently.
The chemical name for water is dihydrogen monoxide, represented as H₂O, comprising two hydrogen atoms and one oxygen atom.
The density of water is 1 gram per cubic centimeter (g/cm³) at its maximum density at 4°C (39.2°F).
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What type of bond holds the hydrogen and oxygen atoms together in a water molecule?
Ionic bond
Covalent bond
Metallic bond
Hydrogen bond
What is the angle between the hydrogen-oxygen-hydrogen atoms in a water molecule?
90 degrees
104.5 degrees
120 degrees
180 degrees
At what temperature does water reach its maximum density?
0°C
4°C
25°C
100°C
What is the boiling point of water at standard atmospheric pressure?
0°C
50°C
100°C
150°C
What is the pH of pure water at 25°C?
3
5
7
9
What is the main reason for the high surface tension of water?
Ionic bonds
Hydrogen bonds
Covalent bonds
Metallic bonds
Which process describes the transition of water from a liquid to a gas?
Melting
Freezing
Condensation
Evaporation
Which property of water is primarily responsible for its role in temperature regulation in organisms?
Low density
High specific heat capacity
High viscosity
Low boiling point
What term describes the attraction between water molecules and other types of molecules?
Cohesion
Adhesion
Surface tension
Viscosity
What is the freezing point of water at standard atmospheric pressure?
0°C
-10°C
-50°C
10°C
Before you leave, take our quick quiz to enhance your learning!

