What is the atomic number of xenon?
52
54
56
58
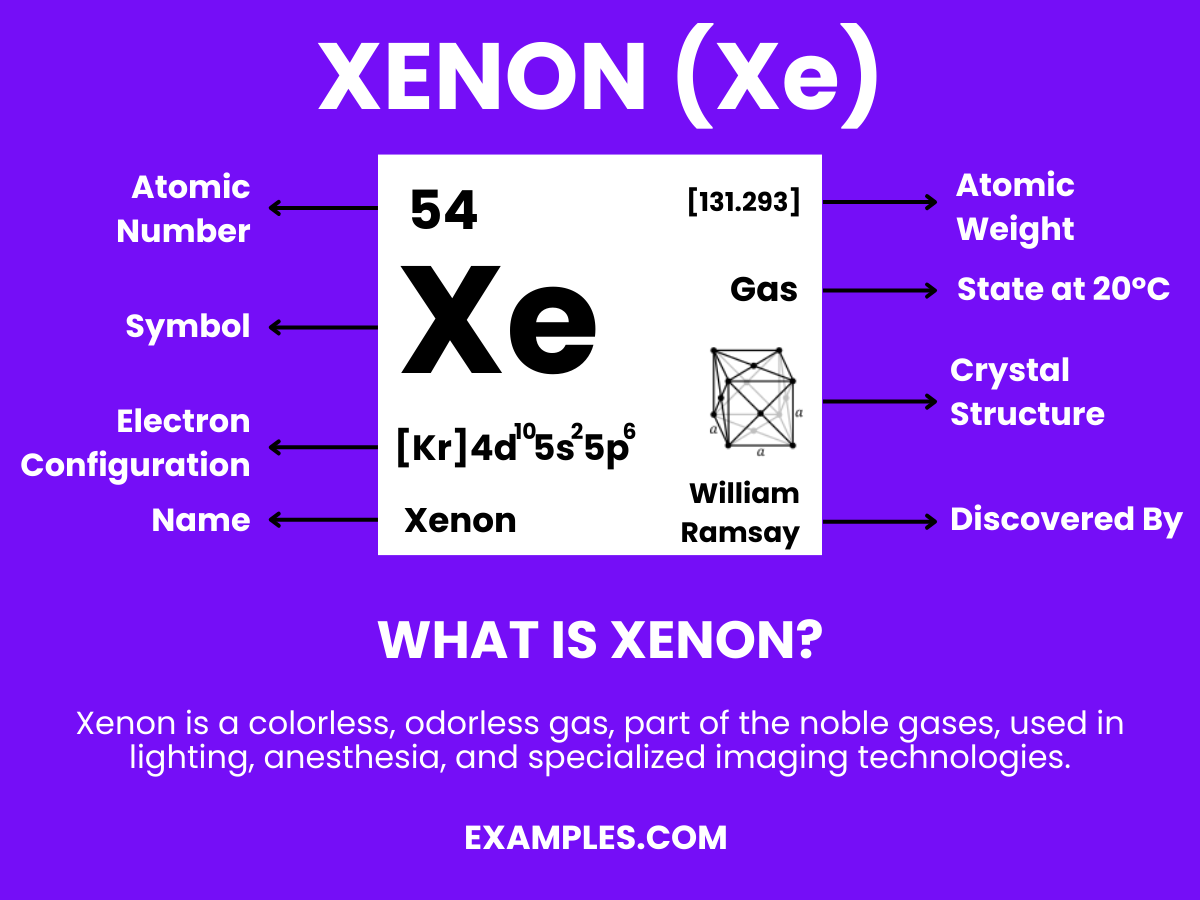
Xenon, a noble gas known for its rare and inert nature, illuminates the world in ways beyond just bright lights. As a teacher, understanding Xenon can enrich your science curriculum with fascinating examples of its uses in lighting, medical imaging, and even propulsion systems. Dive into this complete guide to discover the intriguing world of Xenon, its properties, and its applications. Engage students with real-life examples and tips on how Xenon is revolutionizing various fields.
Xenon is a colorless, dense, odorless noble gas found in the Earth’s atmosphere in trace amounts. As a noble gas, it is chemically inert, not reacting with other elements under standard conditions. It’s widely used in light-emitting devices due to its brilliant emissions and has applications in medical imaging and satellite propulsion. Understanding Xenon is essential for exploring advanced concepts in chemistry and physics, making it a fascinating subject for educators and students alike.
| Helium |
| Neon |
| Argon |
| Krypton |
| Radon |
Formula: Xe
Composition: A single xenon atom.
Bond Type: Generally non-reactive, with a complete valence shell.
Molecular Structure: Monatomic gas.
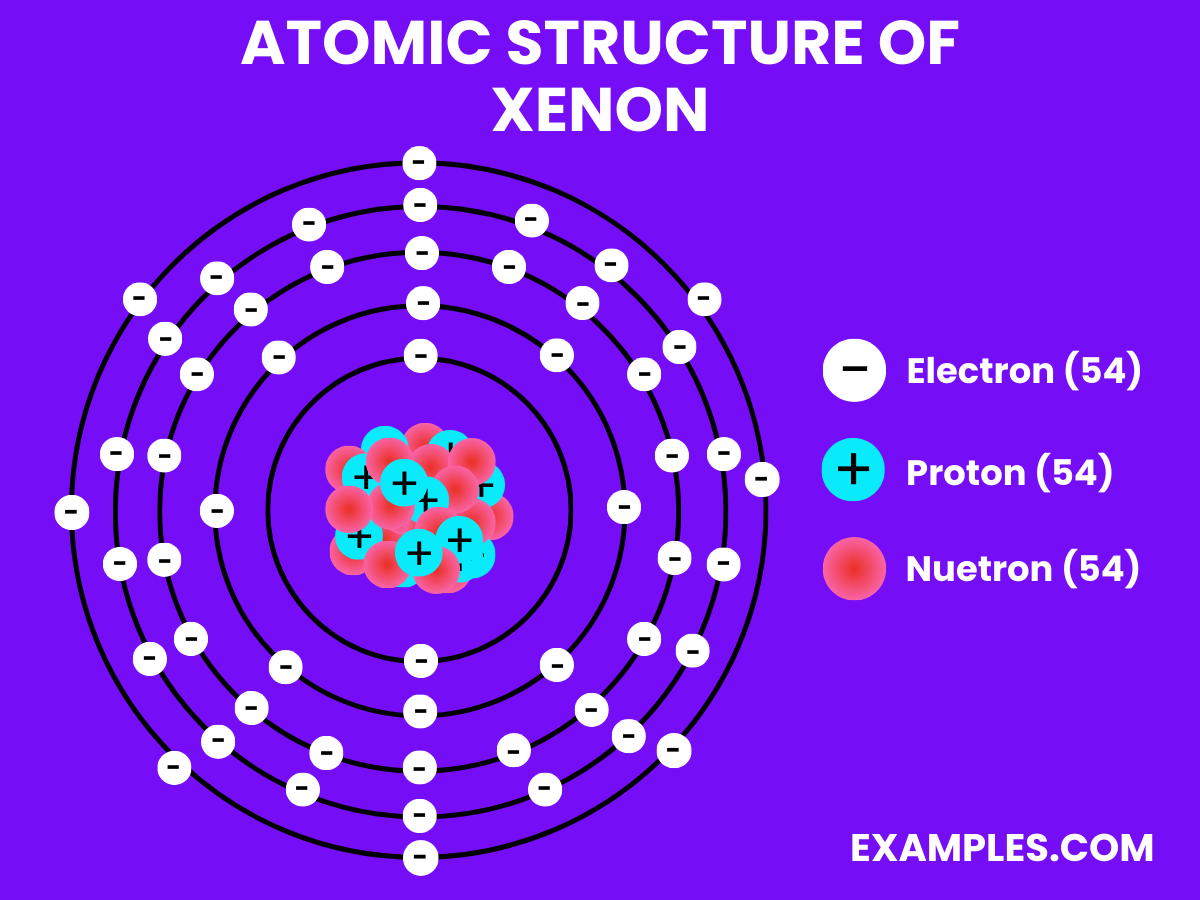
Electron Configuration: 54 electrons; configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶.
Significance: Used in lighting, like high-intensity lamps and xenon flash lamps.
Role in Chemistry: Inert, but forms compounds like xenon difluoride (XeF₂) under extreme conditions.
| Property | Description |
|---|---|
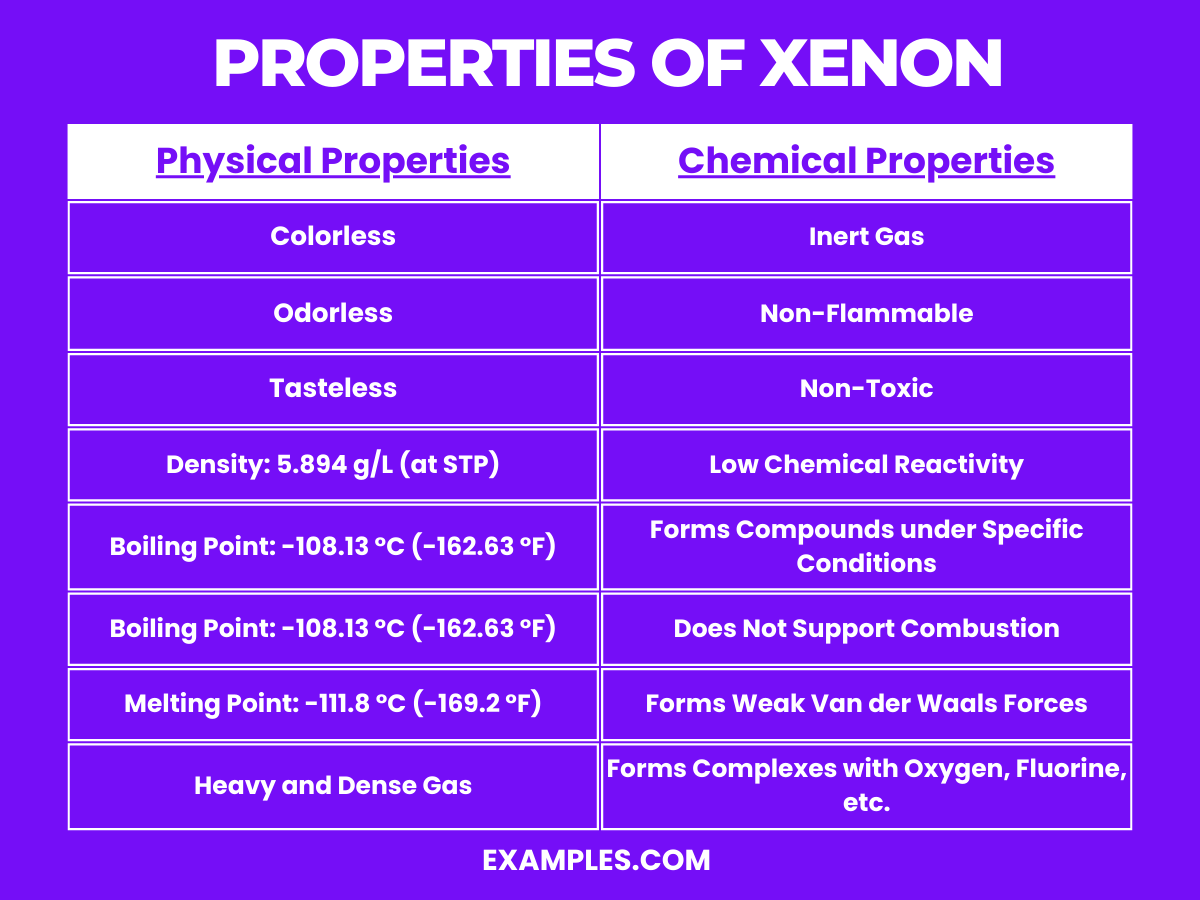
| State at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
| Density | 5.894 g/L at 0°C and 1 atm |
| Boiling Point | -108.13 °C (-162.63 °F) |
| Melting Point | -111.8 °C (-169.2 °F) |
| Gas Density | Heavy and dense compared to other noble gases |
| Property | Value with Unit |
|---|---|
| Boiling Point | -108.1 °C |
| Melting Point | -111.8 °C |
| Critical Temperature | 16.6 °C |
| Critical Pressure | 5.84 MPa |
| Heat of Vaporization | 12.64 kJ/mol |
| Heat of Fusion | 2.27 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.158 J/g·K |
| Thermal Conductivity | 0.00565 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 0°C and 1 atm) | 5.894 kg/m³ (Gas) |
| Viscosity (at 0°C) | 0.021 mPa·s (Gas) |
| Solubility in Water (at 20°C) | 0.1 g/100 mL of water |
| Phase at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless |
| Property | Value with Unit |
|---|---|
| Electrical Conductivity | Non-conductive |
| Electronegativity (Pauling scale) | 2.6 |
| Ionization Energy | First: 12.13 eV |
| Electron Affinity | 0 eV (Practically Non-reactive) |
| Property | Value with Unit |
|---|---|
| Atomic Number | 54 |
| Atomic Mass | 131.293 amu |
| Isotopes | Numerous (stable: ^124Xe, ^126Xe, ^128Xe, ^129Xe, ^130Xe, ^131Xe, ^132Xe, ^134Xe, ^136Xe) |
| Natural Abundance (for ^129Xe) | 26.4% |
| Natural Abundance (for ^132Xe) | 26.9% |
| Nuclear Spin (for ^129Xe) | 1/2 ℏ |
| Nuclear Spin (for ^131Xe) | 3/2 ℏ |
| Neutron Cross Section (for ^129Xe) | 21 barns |
| Neutron Cross Section (for ^131Xe) | 85 barns |
| Nuclear Magnetic Moment (for ^129Xe) | -0.778 µN |
| Nuclear Magnetic Moment (for ^131Xe) | 0.692 µN |
Xenon, a noble gas, forms several intriguing chemical compounds despite its general inertness. Below are the top six compounds of xenon along with their relevant equations:
Xenon has several isotopes, each with unique properties. The table below describes some of the key isotopes of xenon:
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Decay Mode |
|---|---|---|---|---|
| Xe-124 | 124 | 0.095 | 1.8 x 10²² years | Double electron capture |
| Xe-126 | 126 | 0.089 | Stable | – |
| Xe-128 | 128 | 1.92 | Stable | – |
| Xe-129 | 129 | 26.44 | Stable | – |
| Xe-130 | 130 | 4.08 | Stable | – |
| Xe-131 | 131 | 21.18 | Stable | – |
| Xe-132 | 132 | 26.89 | Stable | – |
| Xe-134 | 134 | 10.44 | Stable | – |
| Xe-136 | 136 | 8.87 | 2.165 x 10²¹ years | Double beta decay |
Xenon, a noble gas, is utilized in various applications due to its unique properties. Here are five of the most prominent uses of xenon:
The commercial production of xenon is primarily achieved through the fractional distillation of liquefied air. Here is a simplified overview of the process:
Due to the extensive processing required and the low concentration of xenon in the atmosphere, the production of xenon is relatively expensive compared to other gases. Nonetheless, its unique properties make it highly valuable for the various applications mentioned.
Xenon, as an inert, noble gas, is generally considered to be non-toxic and chemically unreactive. However, it can have certain health effects, particularly when used in medical or industrial settings:
Xenon, being a rare and inert gas, has minimal environmental impact:
Xenon is rare due to its low abundance in Earth’s atmosphere, comprising just 0.0000087%, making it one of the least common elements.
Xenon can react under certain conditions, primarily forming compounds with fluorine and oxygen, despite its general inertness as a noble gas.
Xenon glows blue when electrified because it emits light in the blue spectrum. This is due to electron excitation and subsequent release of photons.
Xenon is important for its unique applications in lighting, medical imaging, anesthesia, space exploration, and nuclear energy research, leveraging its inert and dense properties.
Xenon, a rare and noble gas, plays a crucial role in diverse fields due to its unique properties. From enhancing lighting technology to its use in medical imaging and anesthesia, xenon’s applications highlight its importance. Understanding xenon’s characteristics and reactions offers valuable insights, beneficial for industries and scientific research alike.

Xenon, a noble gas known for its rare and inert nature, illuminates the world in ways beyond just bright lights. As a teacher, understanding Xenon can enrich your science curriculum with fascinating examples of its uses in lighting, medical imaging, and even propulsion systems. Dive into this complete guide to discover the intriguing world of Xenon, its properties, and its applications. Engage students with real-life examples and tips on how Xenon is revolutionizing various fields.
Xenon is a colorless, dense, odorless noble gas found in the Earth’s atmosphere in trace amounts. As a noble gas, it is chemically inert, not reacting with other elements under standard conditions. It’s widely used in light-emitting devices due to its brilliant emissions and has applications in medical imaging and satellite propulsion. Understanding Xenon is essential for exploring advanced concepts in chemistry and physics, making it a fascinating subject for educators and students alike.
Formula: Xe
Composition: A single xenon atom.
Bond Type: Generally non-reactive, with a complete valence shell.
Molecular Structure: Monatomic gas.
Electron Configuration: 54 electrons; configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶.
Significance: Used in lighting, like high-intensity lamps and xenon flash lamps.
Role in Chemistry: Inert, but forms compounds like xenon difluoride (XeF₂) under extreme conditions.


Property | Description |
|---|---|
State at Room Temperature | Gas |
Color | Colorless |
Odor | Odorless |
Taste | Tasteless |
Density | 5.894 g/L at 0°C and 1 atm |
Boiling Point | -108.13 °C (-162.63 °F) |
Melting Point | -111.8 °C (-169.2 °F) |
Gas Density | Heavy and dense compared to other noble gases |
Inert Gas: Xenon is a noble gas, which makes it largely unreactive due to its complete valence electron shell. This inertness defines much of its chemical behavior.
Non-Flammable: As an inert gas, xenon does not support combustion and is non-flammable. It’s often used in environments where preventing reactions is critical.
Non-Toxic: Xenon is non-toxic and poses no significant health risks when inhaled in small amounts. However, in large concentrations, it can cause asphyxiation by displacing oxygen.
Low Chemical Reactivity: While xenon is mostly inert, it is more reactive than other noble gases like helium or neon. Under certain conditions, particularly involving high pressure and the presence of fluorine or oxygen, it can form compounds.
Forms Compounds under Specific Conditions: Xenon can form a variety of chemical compounds, primarily with fluorine and oxygen, such as xenon difluoride (XeF₂), xenon tetrafluoride (XeF₄), xenon hexafluoride (XeF₆), and xenon tetroxide (XeO₄). These compounds are generally colorless solids that are powerful oxidizing agents.
Does Not Support Combustion: Xenon does not support or enhance combustion, maintaining its inertness even in environments that would be reactive for other substances.
Weak Van der Waals Forces: As with other noble gases, xenon molecules are held together by weak van der Waals forces, influencing its physical properties like boiling and melting points.
Forms Complexes with Oxygen, Fluorine, etc.: Xenon can form complex compounds with oxygen, fluorine, and other elements under extreme conditions. This ability is unique among the noble gases and has led to a variety of scientific applications and studies.
Property | Value with Unit |
|---|---|
Boiling Point | -108.1 °C |
Melting Point | -111.8 °C |
Critical Temperature | 16.6 °C |
Critical Pressure | 5.84 MPa |
Heat of Vaporization | 12.64 kJ/mol |
Heat of Fusion | 2.27 kJ/mol |
Specific Heat Capacity (at 25°C) | 0.158 J/g·K |
Thermal Conductivity | 0.00565 W/m·K |
Property | Value with Unit |
|---|---|
Density (at 0°C and 1 atm) | 5.894 kg/m³ (Gas) |
Viscosity (at 0°C) | 0.021 mPa·s (Gas) |
Solubility in Water (at 20°C) | 0.1 g/100 mL of water |
Phase at Room Temperature | Gas |
Color | Colorless |
Odor | Odorless |
Property | Value with Unit |
|---|---|
Electrical Conductivity | Non-conductive |
Electronegativity (Pauling scale) | 2.6 |
Ionization Energy | First: 12.13 eV |
Electron Affinity | 0 eV (Practically Non-reactive) |
Property | Value with Unit |
|---|---|
Atomic Number | 54 |
Atomic Mass | 131.293 amu |
Isotopes | Numerous (stable: ^124Xe, ^126Xe, ^128Xe, ^129Xe, ^130Xe, ^131Xe, ^132Xe, ^134Xe, ^136Xe) |
Natural Abundance (for ^129Xe) | 26.4% |
Natural Abundance (for ^132Xe) | 26.9% |
Nuclear Spin (for ^129Xe) | 1/2 ℏ |
Nuclear Spin (for ^131Xe) | 3/2 ℏ |
Neutron Cross Section (for ^129Xe) | 21 barns |
Neutron Cross Section (for ^131Xe) | 85 barns |
Nuclear Magnetic Moment (for ^129Xe) | -0.778 µN |
Nuclear Magnetic Moment (for ^131Xe) | 0.692 µN |

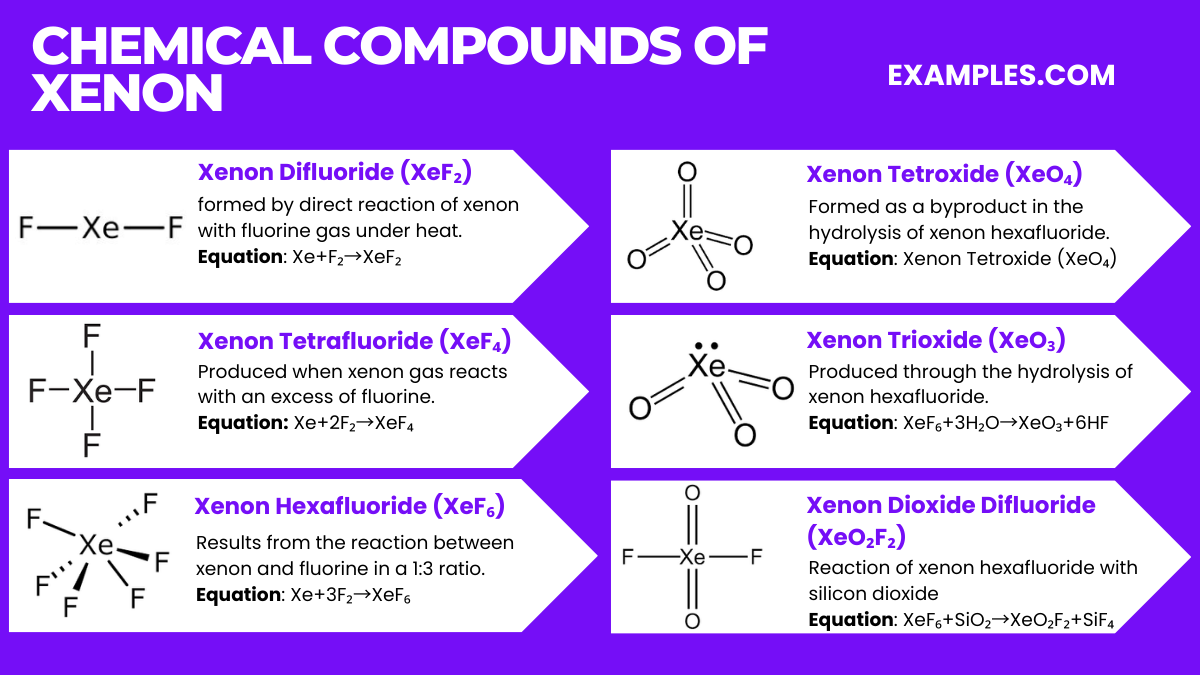
Xenon, a noble gas, forms several intriguing chemical compounds despite its general inertness. Below are the top six compounds of xenon along with their relevant equations:
Xenon Difluoride (XeF₂)
Formation Equation: Xe+F₂→XeF₂
Xenon difluoride is formed by direct reaction of xenon with fluorine gas under heat.
Xenon Tetrafluoride (XeF₄)
Formation Equation: Xe+2F₂→XeF₄
This compound is produced when xenon gas reacts with an excess of fluorine.
Xenon Hexafluoride (XeF₆)
Formation Equation: Xe+3F₂→XeF₆
Xenon hexafluoride results from the reaction between xenon and fluorine in a 1:3 ratio.
Xenon Tetroxide (XeO₄)
Formation Equation: XeO₆+2H₂O→XeO₄+4HF
Xenon tetroxide is formed as a byproduct in the hydrolysis of xenon hexafluoride.
Xenon Trioxide (XeO₃)
Formation Equation: XeF₆+3H₂O→XeO₃+6HF
This compound is produced through the hydrolysis of xenon hexafluoride.
Xenon Dioxide Difluoride (XeO₂F₂)
Formation Equation: XeF₆+SiO₂→XeO₂F₂+SiF₄
The reaction of xenon hexafluoride with silicon dioxide yields xenon dioxide difluoride.
Xenon has several isotopes, each with unique properties. The table below describes some of the key isotopes of xenon:
Isotope | Mass Number | Natural Abundance (%) | Half-Life | Decay Mode |
|---|---|---|---|---|
Xe-124 | 124 | 0.095 | 1.8 x 10²² years | Double electron capture |
Xe-126 | 126 | 0.089 | Stable | – |
Xe-128 | 128 | 1.92 | Stable | – |
Xe-129 | 129 | 26.44 | Stable | – |
Xe-130 | 130 | 4.08 | Stable | – |
Xe-131 | 131 | 21.18 | Stable | – |
Xe-132 | 132 | 26.89 | Stable | – |
Xe-134 | 134 | 10.44 | Stable | – |
Xe-136 | 136 | 8.87 | 2.165 x 10²¹ years | Double beta decay |

Xenon, a noble gas, is utilized in various applications due to its unique properties. Here are five of the most prominent uses of xenon:
Lighting: Xenon is used in high-intensity discharge lamps, particularly in automobile headlights and projector lamps. These lamps produce light that closely resembles natural daylight. Xenon gas, when electrified, emits a bright, white light, making it ideal for these applications.
Medical Imaging: In medical imaging, particularly in computed tomography (CT) scans, xenon is used as a contrast agent. Its high atomic number makes it effective in enhancing the contrast of images, particularly in visualizing the lungs and brain. This helps in providing clearer images for better diagnosis.
Anesthesia: Xenon is used as a general anesthetic. It is preferred in certain cases due to its rapid onset and minimal side effects. Unlike other anesthetics, xenon is not metabolized in the body and is non-toxic, making it safer for patients with certain conditions.
Space Exploration: In ion propulsion engines, used in spacecraft, xenon is a preferred propellant. Its inert nature and high atomic mass make it effective for this purpose. When ionized, xenon atoms can be accelerated to high speeds, providing thrust for spacecraft.
Nuclear Energy Research: Xenon is used in nuclear energy research, particularly in bubble chambers, neutron detectors, and as a part of nuclear fuel rods. Its properties enable it to detect very small particles and interactions, making it valuable in experimental physics.
The commercial production of xenon is primarily achieved through the fractional distillation of liquefied air. Here is a simplified overview of the process:
Air Liquefaction: The first step involves cooling and compressing air until it liquefies. This is usually done in large-scale air separation units (ASUs).
Fractional Distillation: The liquefied air is then subjected to fractional distillation. In this process, the air is gradually warmed in a distillation column. Different components of air have different boiling points and thus separate at various levels of the column.
Isolation of Xenon: Xenon, being one of the heavier components, is collected towards the bottom of the distillation column. However, since xenon is present in very low concentrations in the atmosphere (about 0.0000087% by volume), large volumes of air are required to extract a significant amount of xenon.
Purification: The collected xenon may still contain traces of other gases, and further purification steps are taken. This could involve additional distillation steps, absorption methods, or other chemical processes to ensure high purity of the xenon gas.
Due to the extensive processing required and the low concentration of xenon in the atmosphere, the production of xenon is relatively expensive compared to other gases. Nonetheless, its unique properties make it highly valuable for the various applications mentioned.
Xenon, as an inert, noble gas, is generally considered to be non-toxic and chemically unreactive. However, it can have certain health effects, particularly when used in medical or industrial settings:
As an Anesthetic: Xenon is used as a general anesthetic in surgical procedures. It is known for its rapid onset and recovery times, and minimal side effects. Unlike other anesthetics, it doesn’t typically cause nausea or postoperative complications. However, as with all anesthetics, there’s a risk of adverse reactions, particularly in patients with certain medical conditions.
Inhalation Risks: In high concentrations, xenon can displace oxygen in the air, potentially leading to oxygen deprivation, known as hypoxia. Symptoms of hypoxia can include dizziness, headache, and in severe cases, loss of consciousness. It is important to ensure proper ventilation when handling xenon in confined spaces.
Effect on Blood Pressure: Xenon has been observed to increase blood pressure in some individuals when used as an anesthetic. This effect is typically transient and managed during medical procedures, but it underscores the need for careful monitoring during its use.
Minimal Metabolic Interaction: A notable advantage of xenon is that it is minimally metabolized in the body and does not produce significant metabolites. This reduces the risk of toxicity or adverse reactions related to metabolic processes.
Allergic Reactions: There is minimal risk of allergic reactions to xenon due to its inert nature. This makes it a safer option compared to other anesthetics that may trigger allergies.
Xenon, being a rare and inert gas, has minimal environmental impact:
Non-reactive Nature: Xenon does not react with other elements or compounds under normal environmental conditions. This inertness means it does not contribute to air or water pollution.
No Greenhouse Gas Effect: Unlike some other gases, xenon does not contribute to the greenhouse gas effect. It doesn’t absorb infrared radiation, which is a key factor in global warming.
No Ozone Depletion: Xenon does not affect the ozone layer. It lacks the chemical properties necessary to interact with or break down ozone molecules.
Limited Availability: The rarity of xenon in the Earth’s atmosphere (approximately 0.0000087% of the atmosphere by volume) means that its industrial and medical use has a negligible impact on its overall concentration in the environment.
Safe Disposal: Being non-toxic and non-reactive, xenon can be safely released into the atmosphere after use. It does not require special disposal methods to mitigate environmental impact.
Xenon is rare due to its low abundance in Earth’s atmosphere, comprising just 0.0000087%, making it one of the least common elements.
Xenon can react under certain conditions, primarily forming compounds with fluorine and oxygen, despite its general inertness as a noble gas.
Xenon glows blue when electrified because it emits light in the blue spectrum. This is due to electron excitation and subsequent release of photons.
Xenon is important for its unique applications in lighting, medical imaging, anesthesia, space exploration, and nuclear energy research, leveraging its inert and dense properties.
Xenon, a rare and noble gas, plays a crucial role in diverse fields due to its unique properties. From enhancing lighting technology to its use in medical imaging and anesthesia, xenon’s applications highlight its importance. Understanding xenon’s characteristics and reactions offers valuable insights, beneficial for industries and scientific research alike.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of xenon?
52
54
56
58
Xenon is a:
Metal
Metalloid
Noble gas
Non-metal
Which of the following is a common use of xenon?
In light bulbs
As a structural material
In chemical synthesis
As a catalyst
Xenon was discovered in which year?
1889
1898
1905
1912
What is the primary method of obtaining xenon?
Mining
Fractional distillation of air
Chemical synthesis
Electrolysis of water
Which of the following properties is NOT true for xenon?
It is a good conductor of electricity
It is colorless
It is odorless
It is heavy
Xenon is most commonly found in:
The Earth\'s crust
The Earth\'s atmosphere
The ocean
The human body
Which of the following isotopes of xenon is used in medical imaging?
Xenon-129
Xenon-131
Xenon-134
Xenon-136
Xenon reacts with fluorine to form:
Xenon difluoride
Xenon tetrafluoride
Xenon hexafluoride
All of the above
Xenon is a:
Solid at room temperature
Liquid at room temperature
Gas at room temperature
Plasma at room temperature
Before you leave, take our quick quiz to enhance your learning!

