What is the atomic number of Ytterbium?
69
71
70
72
On an enlightening exploration of Ytterbium, a fascinating element with a silver gleam that hides a wealth of applications beneath its modest exterior. This complete guide illuminates Ytterbium’s essential characteristics, from its definition and fundamental meaning to its myriad uses in technology and science. We’ll delve into its various compounds, providing examples that showcase Ytterbium’s versatility and pivotal role in advancing modern innovations. Whether you’re a seasoned scientist or simply curious about the elements, this guide to Ytterbium offers a rich, keyword-enriched narrative designed to enhance your understanding and appreciation of this remarkable element.
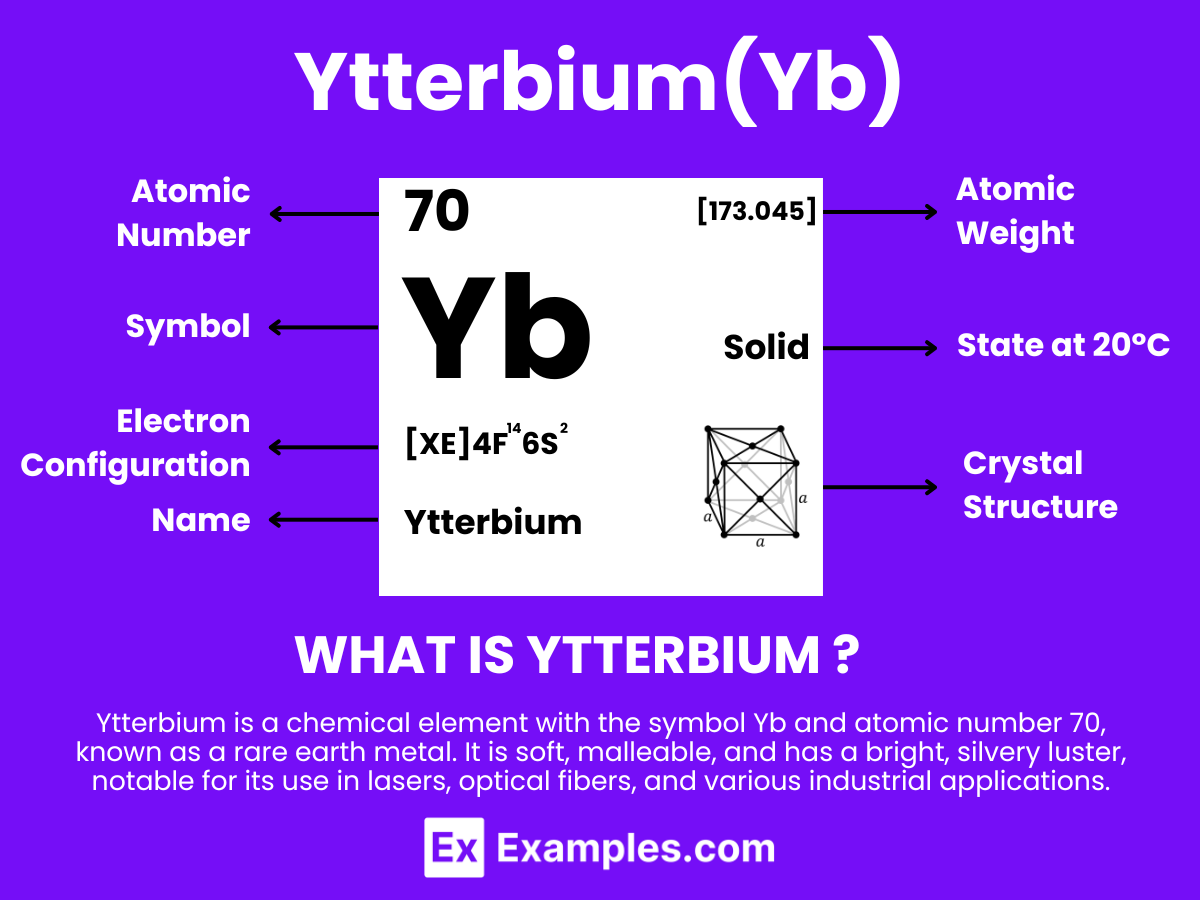
Ytterbium is a chemical element with the symbol Yb and atomic number 70. It belongs to the lanthanide series, a subset of the rare earth elements, in the periodic table. Ytterbium is a soft, malleable, and ductile metal with a bright, silvery luster. It exhibits a relatively high density and is stable in air due to the formation of a surface oxide layer that prevents further oxidation.
In its natural state, ytterbium is found in several minerals, including xenotime, monazite, and euxenite, usually in small amounts. It is not found in a free state in nature. The element was discovered by the Swiss chemist Jean Charles Galissard de Marignac in 1878 through his work on erbium oxide.
Ytterbium, in contrast to hydrogen, is a metallic element with characteristics that reflect its position within the lanthanide series of the periodic table. Its properties include a metallic luster, malleability, and conductivity, which significantly diverge from the gaseous nature and simple molecular formation of hydrogen. Ytterbium’s behavior at the atomic and molecular levels is influenced by its electronic structure and its role in various chemical and physical processes.
Atomic Level: Each ytterbium atom (Yb) contains 70 protons in its nucleus and is expected to have 70 electrons orbiting around it. The electron configuration of ytterbium is predicted to be [Xe] 4f¹⁴ 6s², indicating a completed 4f shell and suggesting stability and a low level of chemical reactivity compared to other elements. This stable electron configuration leads to ytterbium’s common +2 and +3 oxidation states, with the +3 state being more prevalent and chemically active.
Molecular Formation: Unlike hydrogen, which forms simple diatomic molecules (H₂) through covalent bonding, ytterbium, due to its metallic nature, would not form molecules in a similar manner. In its solid form, ytterbium is expected to exhibit a metallic lattice structure typical of metals. This structure involves metallic bonding, where electrons are delocalized over many ytterbium atoms, differing fundamentally from the discrete electron sharing seen in hydrogen’s covalent bonds. The crystalline structure of solid ytterbium is usually face-centered cubic (fcc), common among some rare earth metals
| Property | Value |
|---|---|
| Atomic Number | 70 |
| Atomic Weight | 173.04 g/mol |
| Density | 6.90 g/cm³ |
| Melting Point | 824 °C |
| Boiling Point | 1196 °C |
| State at 20°C | Solid |
| Color | Silvery-white |
Ytterbium is a rare earth metal with interesting chemical properties, predominantly existing in the +2 and +3 oxidation states, though the +3 state is more stable and common. It reacts slowly with water and quickly with acids to form ytterbium salts. Below are examples with relevant equations:
Ytterbium reacts with water to form ytterbium hydroxide and hydrogen gas, though this reaction is less vigorous compared to other reactive metals.
Ytterbium dissolves in hydrochloric acid, forming ytterbium chloride and hydrogen gas.
| Property | Value |
|---|---|
| Standard Molar Entropy (S°298) | 49.7 J/(mol·K) |
| Heat of Fusion | 7.66 kJ/mol |
| Heat of Vaporization | 159 kJ/mol |
| Thermal Conductivity | 38.5 W/(m·K) |
| Specific Heat Capacity | 26.74 J/(mol·K) |
| Thermal Expansion | 26.3 µm/(m·K) |
| Debye Temperature | 129 K |
| Property | Value |
|---|---|
| Young’s Modulus | 23.9 GPa |
| Shear Modulus | 9.9 GPa |
| Bulk Modulus | 30.5 GPa |
| Poisson’s Ratio | 0.207 |
| Mohs Hardness | 2-3 |
| Vickers Hardness | 345 MPa |
| Brinell Hardness | 343 MPa |
| Electrical Resistivity | 0.250 µΩ·m (at 20 °C) |
| Property | Description |
|---|---|
| Electronic Configuration | [Xe] 4f¹⁴ 6s² |
| Magnetic Ordering | Paramagnetic |
| Electrical Conductivity | Good conductor of electricity |
| Optical Properties | Ytterbium ions can be used in lasers for their ability to absorb and emit light efficiently |
| Superconductivity | Exhibits superconductivity under certain low-temperature conditions |
| Property | Description |
|---|---|
| Natural Isotopes | Yb-168, Yb-170, Yb-171, Yb-172, Yb-173, Yb-174, and Yb-176 |
| Radioactive Isotopes | Yb-169, Yb-175, and others with shorter half-lives |
| Stable Isotopes | Yb-168, Yb-170, Yb-171, Yb-172, Yb-173, Yb-174, and Yb-176 |
| Neutron Cross Section | Yb-174 has a high neutron absorption cross-section, useful in certain nuclear applications |
| Isotopic Abundance | Ytterbium has a natural mix of isotopes, with Yb-174 being the most abundant in nature |
| Half-lives | Ranges from days for the lightest isotopes to potentially stable for the heavier isotopes |
The preparation of ytterbium typically involves several steps, starting from its ores, such as monazite and xenotime, which contain a mixture of rare earth elements. The process includes:
Ytterbium forms a variety of compounds, mainly in the +3 oxidation state. Below are some key ytterbium compounds, their preparation equations, and properties:
| Isotope | Natural Abundance (%) | Half-Life | Decay Mode |
|---|---|---|---|
| Yb-168 | 0.13 | Stable | – |
| Yb-170 | 3.04 | Stable | – |
| Yb-171 | 14.28 | Stable | – |
| Yb-172 | 21.83 | Stable | – |
| Yb-173 | 16.13 | Stable | – |
| Yb-174 | 31.83 | Stable | – |
| Yb-176 | 12.76 | Stable | – |
| Yb-169 | Trace | 32.026 days | Electron capture |
| Yb-175 | Trace | 4.185 days | Beta decay |
| Yb-177 | Trace | 1.911 hours | Beta decay |
Ytterbium, a rare earth element with the symbol Yb and atomic number 70, is not found free in nature but is contained in several minerals, including monazite, xenotime, and euxenite. The production of ytterbium typically involves several steps, starting from the mining of ytterbium-containing minerals.
Ytterbium has a range of applications due to its unique properties, including its ability to emit infrared light, its high density, and its electrical conductivity. Some of its notable applications include:
Article extensively covers ytterbium, from its fundamental isotopes to its myriad uses across technology, medicine, and science. Through detailed exploration of its preparation, chemical compounds, and unique properties, we’ve showcased ytterbium’s integral role in enhancing lasers, optical fibers, and even quantum computing. Ytterbium emerges as a pivotal element, driving innovation and advancing research in multiple disciplines, demonstrating its versatility and essential contribution to modern technology.
Text prompt
Add Tone
Ytterbium Formula
Atomic Structure of Ytterbium
What is the atomic number of Ytterbium?
69
71
70
72
What is the chemical symbol for Ytterbium?
Yb
Yt
Y
Yttr
Ytterbium belongs to which series in the periodic table?
Alkali metals
Transition metals
Lanthanides
Actinides
Which of the following is the most stable oxidation state of Ytterbium?
+1
+2
+3
+4
Ytterbium is named after which place?
A city in Sweden
A scientist
A Greek god
A type of mineral
Which of the following is a common use for Ytterbium?
Jewelry
Nuclear reactors
Medical imaging
Strengthening stainless steel
What is the atomic mass of Ytterbium?
172.04 u
174.04 u
176.04 u
178.04 u
5
6
7
8
Which of the following isotopes of Ytterbium is commonly used in atomic clocks?
Ytterbium-168
Ytterbium-171
Ytterbium-173
Ytterbium-176
What type of magnetism does Ytterbium exhibit?
Ferromagnetism
Paramagnetism
Diamagnetism
Antiferromagnetism
Before you leave, take our quick quiz to enhance your learning!

