What is the atomic number of Yttrium?
37
38
39
40
Yttrium, a key player in the world of rare earth metals, stands out for its significant contributions to various high-tech and medical applications. This comprehensive guide delves into the essence of Yttrium, exploring its unique properties, diverse uses, and the role it plays in enhancing modern technology and healthcare solutions. From its application in LED screens to its pivotal role in cancer treatments, Yttrium’s versatility and importance cannot be overstated. Discover how Yttrium powers innovation and improves daily life through its remarkable compounds and applications.
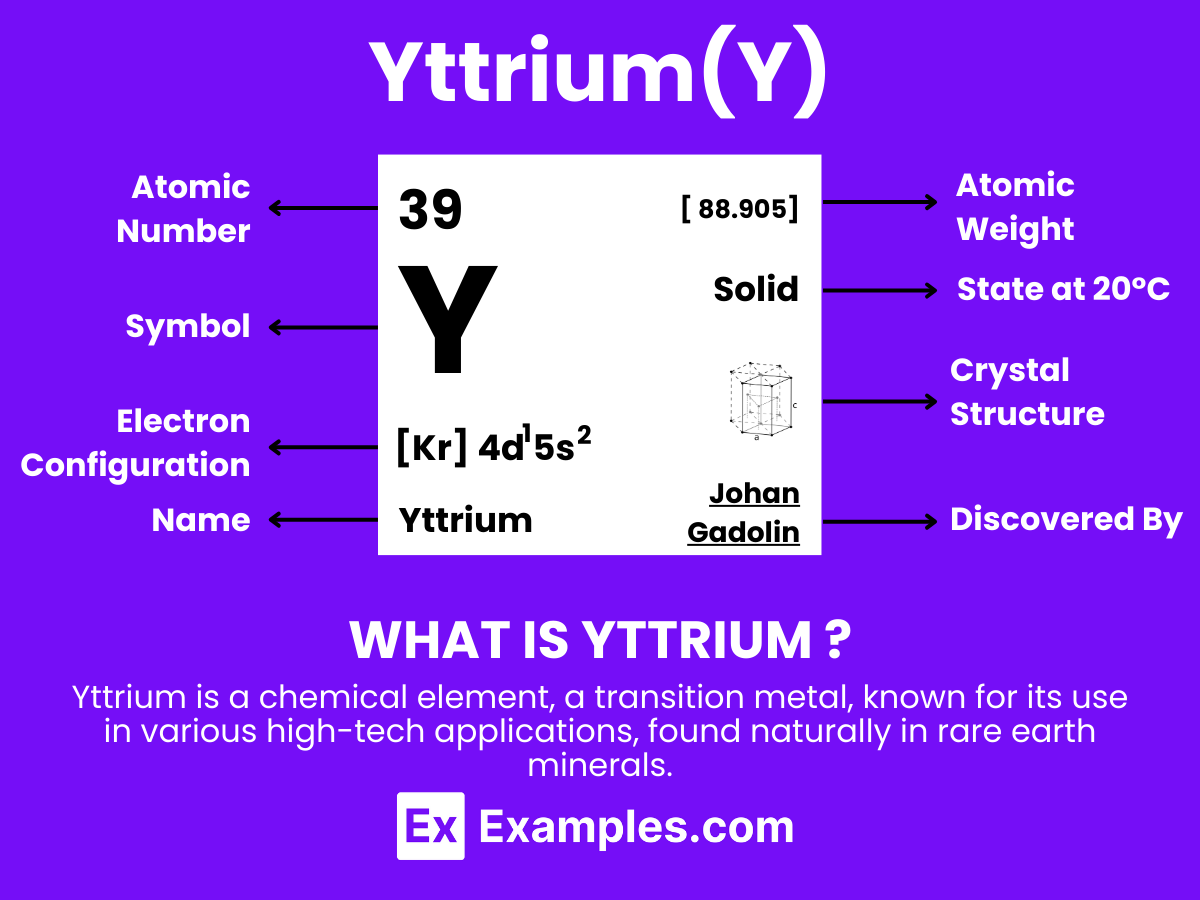
Yttrium is a chemical element with the symbol Y and atomic number 39. It’s a silvery-metallic transition metal that is part of the rare earth group of elements. Despite being less familiar than other metals, yttrium is crucial in modern technology. It’s used in making phosphors for color TV screens, LEDs, and other light-emitting devices. Yttrium is also found in the production of superconductors and in various medical applications, including cancer treatment. This element is not found in its pure form in nature but in mineral compounds such as yttrite and monazite.
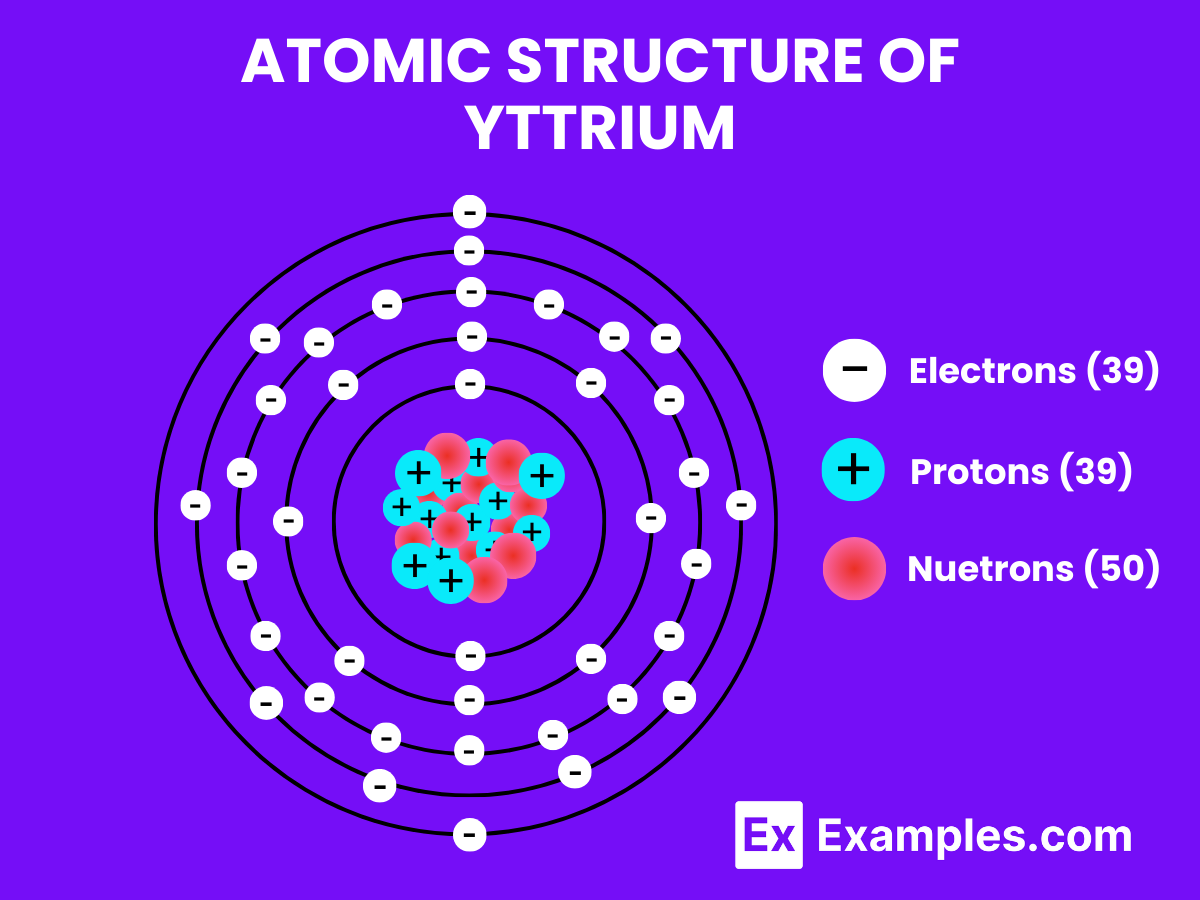
Yttrium, symbolized as Y, is a chemical element with one proton in its nucleus, positioned in the transition metals category of the periodic table. Each yttrium atom has 39 protons in its nucleus and typically 39 electrons, as its atomic number is 39. Yttrium does not form diatomic molecules like hydrogen under normal conditions. Instead, yttrium is more commonly found in various compounds and in solid form due to its metallic nature.
Atomic Level: Each yttrium atom (Y) consists of 39 protons in its nucleus surrounded by 39 electrons.
Molecular Formation: Unlike hydrogen, yttrium does not naturally pair up with another yttrium atom to form a diatomic molecule. In its elemental form, yttrium adopts a crystalline structure, specifically a hexagonal close-packed (hcp) arrangement, which is typical for many transition metals.
Yttrium is known for its use in various applications, including electronics, due to its interesting properties such as enhancing phosphorescence in television screens and LEDs. It is not found in its pure metallic state in nature but usually in the form of minerals like yttrite and xenotime. Yttrium’s compounds, rather than yttrium gas or diatomic molecules, are utilized in industrial and technological applications.
| Property | Description |
|---|---|
| Appearance | Silvery-white and metallic |
| Atomic Number | 39 |
| Atomic Weight | 88.90585 |
| Melting Point | Approximately 1,522°C (2,772°F) |
| Boiling Point | Approximately 3,338°C (6,040°F) |
| Density | 4.472 grams per cubic centimeter at 20°C (solid phase) |
| State at Room Temperature | Solid |
| Electron Configuration | [Kr] 4d¹ 5s² |
| Crystal Structure | Hexagonal close-packed (hcp) at room temperature |
| Thermal Conductivity | 17.2 W/(m·K) |
| Electrical Resistivity | About 596 nΩ·m at 20°C |
| Electronegativity | 1.22 (Pauling scale) |
| Standard Atomic Weight | 88.90585 |
| Phase at STP | Solid |
| Solubility | Insoluble in water, reacts with acids |
| Common Oxidation States | +3 |
Reactivity with Air: Yttrium is fairly reactive in air; it forms a protective oxide layer that prevents further corrosion. Upon heating, it reacts with oxygen to form yttrium oxide (Y₂O₃).
Equation: 4Y+ 3O₂→2Y ₂O₃
Reactivity with Water: Yttrium reacts with water, especially at elevated temperatures, to form yttrium hydroxide H₂).
Equation: 2Y+6H ₂O→2Y(OH)₃+3H₂
Reactivity with Acids: Yttrium dissolves readily in dilute acids, forming solutions of yttrium ions (Y₃+) and hydrogen gas.
Equation: 2Y+6HCl→2YCl₃+3H₂
Reactivity with Halogens: Yttrium reacts with all halogens to form yttrium halides. For example, with chlorine, it forms yttrium chloride (3YCl₃).
Equation: 2Y+3Cl₂→2YCl₃
Formation of Alloys: Yttrium forms alloys with a wide range of metals. These alloys often have enhanced physical properties, such as increased strength and reduced grain size in high-temperature applications.
Complex Formation: Yttrium can form complex ions with various ligands. For example, it forms complexes with fluoride ions, such as YF₆₃, in solution.
Catalytic Properties: Yttrium exhibits catalytic properties in various chemical reactions, including polymerization and the dehydrogenation of hydrocarbons.
| Property | Value with Unit |
|---|---|
| Boiling Point | 3,345 °C |
| Melting Point | 1,522 °C |
| Critical Temperature | Not Available |
| Critical Pressure | Not Available |
| Heat of Vaporization | 365 kJ/mol |
| Heat of Fusion | 11.4 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.298 J/g·K |
| Thermal Conductivity | 17 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at room temperature) | 4,472 kg/m³ |
| Viscosity | Not Applicable (Solid) |
| Solubility | Insoluble in water |
| Color | Silver-metallic |
| Phase at Room Temperature | Solid |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 20°C) | 596 nΩ·m |
| Thermal Conductivity | 17 W/m·K |
| Magnetic Susceptibility | +1.2 × 10^-3 cm^3/mol |
| Electronegativity (Pauling scale) | 1.22 |
| Property | Value with Unit |
|---|---|
| Atomic Number | 39 |
| Atomic Mass | 88.90584 amu |
| Isotopes | ^89Y (100%) |
| Nuclear Spin (for ^89Y) | 1/2 ℏ |
| Neutron Cross Section (for ^89Y) | 1.28 barns |
| Nuclear Magnetic Moment (for ^89Y) | -0.1373 µN |
The preparation of yttrium metal typically involves several steps due to its occurrence in mixed rare earth minerals rather than in a pure form.
Yttrium is most commonly extracted from minerals such as monazite and xenotime, which contain yttrium as well as other rare earth elements. The extraction process begins with the mining of these minerals.
The mined minerals are crushed and then subjected to various physical and chemical processes to increase the concentration of yttrium and separate it from other elements. This often involves the use of solvent extraction or ion exchange techniques.
The concentrated yttrium is usually converted into yttrium oxide (3Y₂O₃) through a calcination process, where the yttrium-containing compounds are heated to decompose them into their oxide forms.
The metallic yttrium is then produced from yttrium oxide using one of several methods:
a. Electrolysis :In this method, yttrium oxide is dissolved in a suitable flux, and then electrolysis is performed to reduce the yttrium ions to metallic yttrium. The electrolysis process typically requires high temperatures.
b. Metallothermic Reduction: Another common method is the metallothermic reduction, where yttrium oxide is reduced to metallic yttrium using a more reactive metal as the reducing agent. Calcium or magnesium is often used for this purpose due to their strong reducing capabilities.
Y₂O3+3Ca→2Y+3CaO
The reaction is carried out in an inert atmosphere to prevent oxidation and contamination.
The yttrium metal produced through electrolysis or metallothermic reduction often contains impurities. It is further refined using techniques such as vacuum distillation or zone melting to obtain high-purity yttrium metal.
Yttrium is often used in alloyed form to improve the properties of other metals. The refined yttrium can be alloyed with other elements depending on the desired application and material properties.
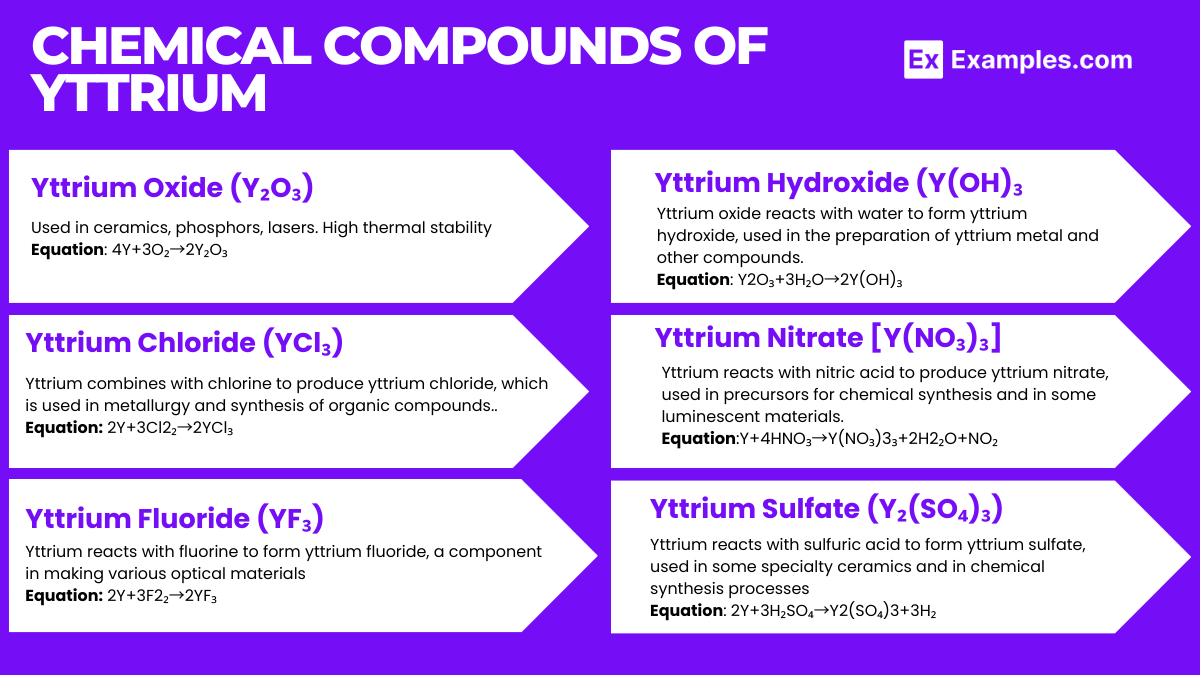
Yttrium, a Group 3 transition metal, forms a variety of chemical compounds that are used across multiple industrial and technological fields. Its compounds are known for their unique chemical and physical properties.
Yttrium oxide is one of the most important yttrium compounds, widely used in ceramics, phosphors, and laser materials due to its high thermal stability and strong luminescence when doped with rare-earth elements.
Formation Equation: 2Y+23O₂→Y₂O₃
YAG is a synthetic crystalline material used as a substrate in laser technology and in the production of various optical components. It serves as a host lattice for doping with different rare-earth ions to produce lasers of various wavelengths.
Synthetic Route: Yttrium oxide reacts with aluminum oxide in a high-temperature furnace to form YAG. 3Y₂O ₃+5Al2O₃→2Y₃Al₅O₁₂
YIG is utilized in microwave filters and is essential in radar and communication devices due to its unique magnetic properties.
Synthetic Route: Similar to YAG, YIG is synthesized by reacting yttrium oxide with iron(III) oxide.3Y2O₃+5Fe₂O₃→2Y₃Fe₅O₂
This compound is known for its high-temperature superconductivity properties. It was one of the first materials discovered to exhibit superconductivity above the boiling point of liquid nitrogen, making it practical for various applications.
General Reaction: The synthesis involves solid-state reactions between yttrium oxide, barium carbonate, and copper oxide.Y₂O₃+4BaCO₃+6CuO→2YBa₂Cu₃O₇−δ+4CO₂
Yttrium fluoride is used in the production of metallic yttrium, fluoride glasses, and in coatings for optical fibers. It is also a component in some dental varnishes for its fluoride-releasing properties.
Formation Equation: Y+2₃F₂→YF₃
This compound is an intermediate in the conversion of yttrium ores into more useful compounds. It can act as a precursor to other yttrium compounds when reacted with appropriate acids or bases.
Formation in Aqueous Solution: Y₃⁺+3OH⁻→Y(OH)₃
These yttrium compounds play crucial roles in modern technology, from improving the efficiency of lasers and LEDs to enabling high-speed communication and groundbreaking medical devices. Their synthesis and applications underscore the importance of yttrium in the advancement of materials science and engineering.
Yttrium naturally occurs as a single isotope, 8989Y is of particular interest for medical applications due to its beta-emitting properties. Here are some details about key yttrium isotopes:
Yttrium has a variety of applications, many of which take advantage of its optical and electronic properties. Here are some of the main uses of yttrium:
The production of yttrium involves several steps, starting from the extraction of yttrium-containing minerals to the purification and production of pure yttrium metal or its compounds. Here’s a general overview:
Yttrium has a wide range of applications across various industries due to its unique properties. Some of the key applications include:
Yttrium, a versatile element, plays a pivotal role in modern technology and industry. From enhancing the durability of ceramics to improving the efficiency of LEDs and superconductors, its applications are vast. The production process, though complex, ensures yttrium’s availability for its critical uses in electronics, medicine, and beyond, showcasing its indispensable contribution to advancement and innovation.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Yttrium?
37
38
39
40
In which group of the periodic table is Yttrium found?
Alkali metals
Transition metals
Alkaline earth metals
Lanthanides
Yttrium is commonly used in the manufacturing of:
Batteries
Ceramics
Fertilizers
Plastics
Which property of Yttrium makes it valuable in LED and LCD screens?
Radioactivity
Phosphorescence
Electrical conductivity
Magnetism
What color does Yttrium emit when used in phosphors?
Red
Blue
Green
Yellow
Yttrium is extracted primarily from which type of ore?
Bauxite
Hematite
Monazite
Galena
The discovery of Yttrium is credited to:
Wilhelm Roentgen
Johan Gadolin
Marie Curie
Antoine Lavoisier
Yttrium-90, an isotope of Yttrium, is used in which field?
Agriculture
Automobile manufacturing
Cancer treatment
Electronics
Yttrium increases the strength of which alloys?
Aluminum alloys
Copper alloys
Nickel alloys
Steel alloys
Which of the following is not a characteristic of Yttrium?
Highly reactive with water
Silver-metallic appearance
Can be stored in oil
Is non-toxic
Before you leave, take our quick quiz to enhance your learning!

