What is the atomic number of Zinc?
28
29
30
31
Discover the fascinating world of Zinc, a versatile element pivotal in numerous fields from healthcare to manufacturing. This comprehensive guide delves deep into Zinc’s properties, its crucial role in human nutrition, its myriad uses in industries, and the intriguing compounds it forms. With practical examples, we illuminate Zinc’s omnipresence in daily life and technological advancements. Embark on a journey to understand Zinc’s essential contributions and its undeniable significance in shaping our modern world.
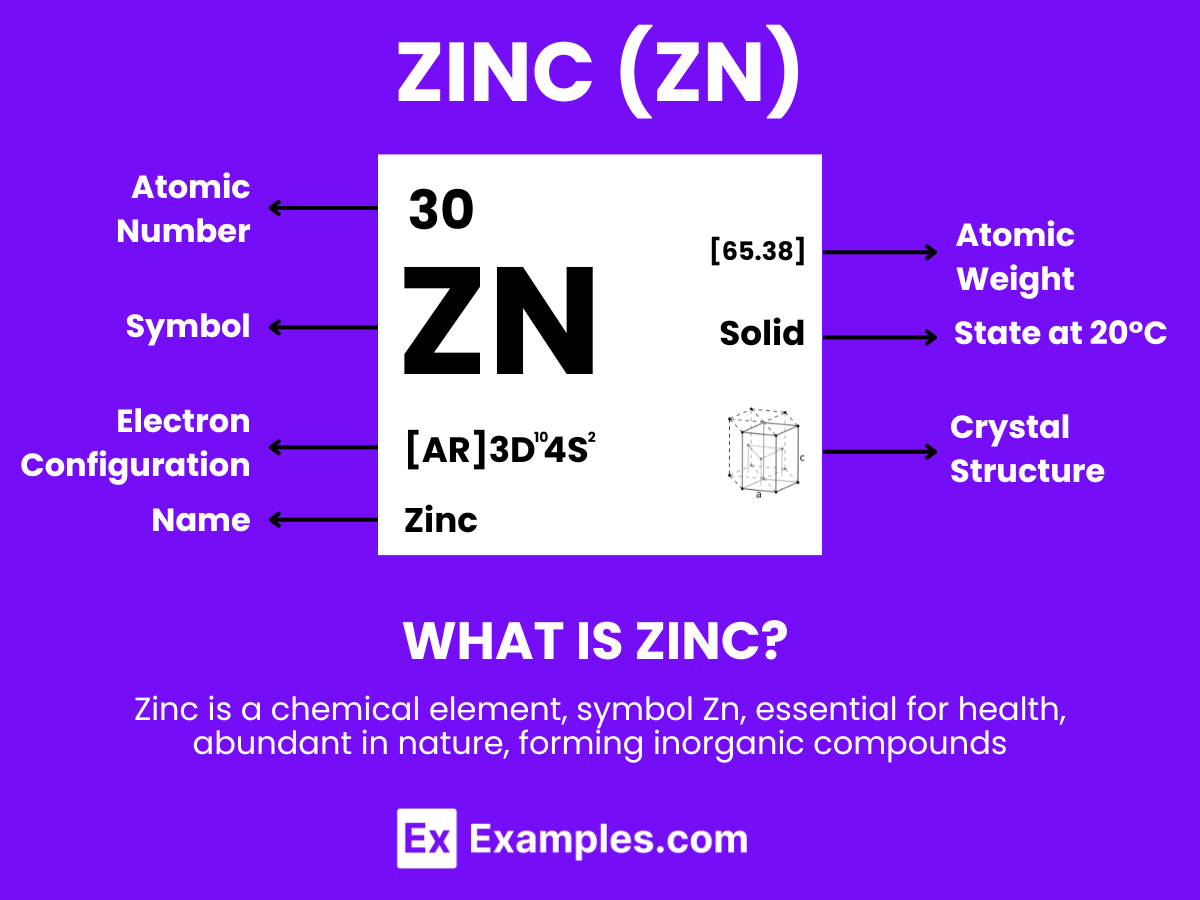
Zinc is a natural element that you find on the periodic table with the symbol “Zn” and atomic number 30. It’s a metal that’s slightly brittle at room temperature but becomes malleable when heated. Zinc is known for its shiny, greyish appearance and is crucial for human health, playing a vital role in immune function, wound healing, and DNA synthesis. Beyond our bodies, it’s widely used in industries to prevent rust on iron and steel products, in making batteries, and in various other applications.
Zinc is a metal known for its critical role in various applications, distinguished by its solid state at room temperature and a notable boiling and melting point that underscores its stability under standard conditions. Zinc’s behavior at the atomic and molecular levels showcases its unique metallic characteristics, driven by its position in the periodic table.
Atomic Level: Each zinc atom (Zn) contains 30 protons in its nucleus, surrounded by 30 electrons. The electron configuration of zinc is [Ar] 3d¹⁰ 4s², indicating it has two electrons in its outermost shell that are available for bonding.
Molecular Formation: In its metallic state, zinc does not form simple molecules as hydrogen does. Instead, zinc atoms are arranged in a crystalline lattice structure in the solid state. This structure is characterized by the sharing of electrons among numerous zinc atoms in a metallic bond, distinct from the covalent bonds found in hydrogen molecules. Upon melting, zinc transitions into a liquid while still maintaining its metallic bonding characteristics to a certain extent, which is evident in its relatively high density and surface tension in the liquid state.
The bonds within zinc’s lattice are sufficiently robust to preserve its structure until reaching its melting point of about 419.53°C (787.15°F). Contrary to hydrogen, which is gaseous at room temperature, zinc remains in a solid state but melts into a liquid at temperatures well above room temperature. It does not naturally occur as a diatomic gas or in a gaseous state under standard conditions due to its high boiling point of approximately 907°C (1665°F).
| Property | Value |
|---|---|
| Appearance | Silvery-grey |
| State at Room Temperature | Solid |
| Melting Point | 419.5°C (787°F) |
| Boiling Point | 907°C (1665°F) |
| Density | 7.14 g/cm³ |
| Molar Mass | 65.38 g/mol |
| Atomic Number | 30 |
| Crystal Structure | Hexagonal Close-Packed (HCP) |
| Property | Value |
|---|---|
| Melting Point | 419.53°C (787.15°F) |
| Boiling Point | 907°C (1665°F) |
| Heat of Fusion | 7.32 kJ/mol |
| Heat of Vaporization | 115 kJ/mol |
| Specific Heat Capacity | 25.470 J/(mol·K) at 25°C |
| Property | Value |
|---|---|
| Density | 7.14 g/cm³ at 20°C |
| Young’s Modulus | 108 GPa |
| Shear Modulus | 43 GPa |
| Bulk Modulus | 70 GPa |
| Mohs Hardness | 2.5 |
| Property | Value |
|---|---|
| Electrical Conductivity | 16.6 × 10⁶ S/m at 20°C |
| Thermal Conductivity | 116 W/(m·K) at 300 K |
| Magnetic Susceptibility | −0.153 × 10⁻⁶ cm³/mol |
| Property | Value |
|---|---|
| Natural Isotopes | ⁶⁴Zn, ⁶⁶Zn, ⁶⁷Zn, ⁶⁸Zn, ⁷⁰Zn |
| Abundance of Stable Isotopes | ⁶⁴Zn: 48.6%, ⁶⁶Zn: 27.9%, ⁶⁷Zn: 4.1%, ⁶⁸Zn: 18.8%, ⁷⁰Zn: 0.6% |
| Cross Section for Thermal Neutrons | 1.1 barns (for ⁶⁴Zn) |
| Radioactive Isotopes | ⁶⁵Zn (half-life: 244 days) |
The preparation of zinc primarily involves the extraction and refining processes to produce pure zinc metal from its ores. The most common ore used for zinc production is zinc blende (sphalerite, ZnS). The preparation process typically includes several key steps:
| Property | Value |
|---|---|
| Melting Point | 419.53°C (787.15°F) |
| Boiling Point | 907°C (1665°F) |
| Heat of Fusion | 7.32 kJ/mol |
| Heat of Vaporization | 115 kJ/mol |
| Specific Heat Capacity | 25.470 J/(mol·K) at 25°C |
| Property | Value |
|---|---|
| Density | 7.14 g/cm³ at 20°C |
| Young’s Modulus | 108 GPa |
| Shear Modulus | 43 GPa |
| Bulk Modulus | 70 GPa |
| Mohs Hardness | 2.5 |
| Property | Value |
|---|---|
| Electrical Conductivity | 16.6 × 10⁶ S/m at 20°C |
| Thermal Conductivity | 116 W/(m·K) at 300 K |
| Magnetic Susceptibility | −0.153 × 10⁻⁶ cm³/mol |
| Property | Value |
|---|---|
| Electrical Conductivity | 16.6 × 10⁶ S/m at 20°C |
| Thermal Conductivity | 116 W/(m·K) at 300 K |
| Magnetic Susceptibility | −0.153 × 10⁻⁶ cm³/mol |
| Isotope | Mass Number | Natural Abundance (%) | Half-life | Notes |
|---|---|---|---|---|
| Zn-64 | 64 | 48.63 | Stable | – |
| Zn-66 | 66 | 27.90 | Stable | – |
| Zn-67 | 67 | 4.10 | Stable | – |
| Zn-68 | 68 | 18.75 | Stable | – |
| Zn-70 | 70 | 0.62 | Stable | Least abundant stable isotope |
| Zn-65 | 65 | – | 244 days | Radioactive, used in research |
| Zn-72 | 72 | – | 46.5 hours | Radioactive, used in medicine |
Zinc, a versatile metal, finds applications across various industries due to its unique properties, such as corrosion resistance, electrochemical compatibility, and ability to alloy with other metals. Some of the primary uses of zinc include:
The production of zinc primarily involves the extraction from its ore, zinc blende (sphalerite), through various processes. The key steps in zinc production include:
This process results in the production of zinc metal, which can then be further refined and used in various applications.
Zinc is a versatile metal with a wide range of applications due to its unique properties, such as corrosion resistance, electrochemical capabilities, and biological importance. Some of the major applications of zinc include:
zinc’s versatile properties and widespread applications make it an invaluable metal in various industries, from construction and manufacturing to healthcare and agriculture. Understanding the thermodynamic, material, electromagnetic, and nuclear properties of zinc enhances our ability to harness its full potential, underscoring its significance in advancing technology and improving daily life.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Zinc?
28
29
30
31
What is the chemical symbol for Zinc?
Zn
Zc
Z
Zi
Zinc is primarily extracted from which ore?
Hematite
Bauxite
Sphalerite
Galena
Which property of Zinc makes it useful for galvanizing steel?
High melting point
Low density
Corrosion resistance
High reflectivity
Zinc oxide is commonly used in which type of product?
Fertilizers
Sunscreens
Detergents
Paints
Which alloy contains Zinc and is used in die casting?
Brass
Bronze
Steel
Zinc-aluminum alloy
What type of bond is found in Zinc metal?
Covalent bond
Ionic bond
Metallic bond
Hydrogen bond
Which of the following is a major application of Zinc in the automotive industry?
Battery production
Tire manufacturing
Engine lubricants
Coating of steel parts
What is the melting point of Zinc?
419.5°C
800.0°C
961.8°C
1200.0°C
Zinc sulfate is commonly used in which field?
Agriculture
Textile manufacturing
Plastic production
Glass making
Before you leave, take our quick quiz to enhance your learning!

