Acid/base Equilibria

- Notes
Acid/base equilibria explore the balance between acids and bases in chemical solutions, a concept crucial to understanding biochemical processes and physiological systems. It involves key principles such as pH, pKa, and the dissociation of acids and bases, alongside the behavior of buffers that resist pH changes. This topic also covers the application of the Henderson-Hasselbalch equation, titration curves, and indicators used to detect equivalence points.
Learning Objectives
In studying “Acid/Base Equilibria” for the MCAT, you should understand key concepts, including pH, pKa, and pKb, and how they relate to acid and base strength. Analyze the behavior of strong and weak acids/bases in equilibrium and apply the Henderson-Hasselbalch equation to buffer systems. Evaluate titration curves, equivalence points, and the role of indicators in acid-base reactions. Explore the application of Le Chatelier’s principle to predict equilibrium shifts. Additionally, develop skills to interpret experimental data, solve pH-related problems, and assess buffer efficiency in MCAT practice passages, focusing on how acid/base chemistry is used in biological and clinical contexts.
Acid and Base Definitions
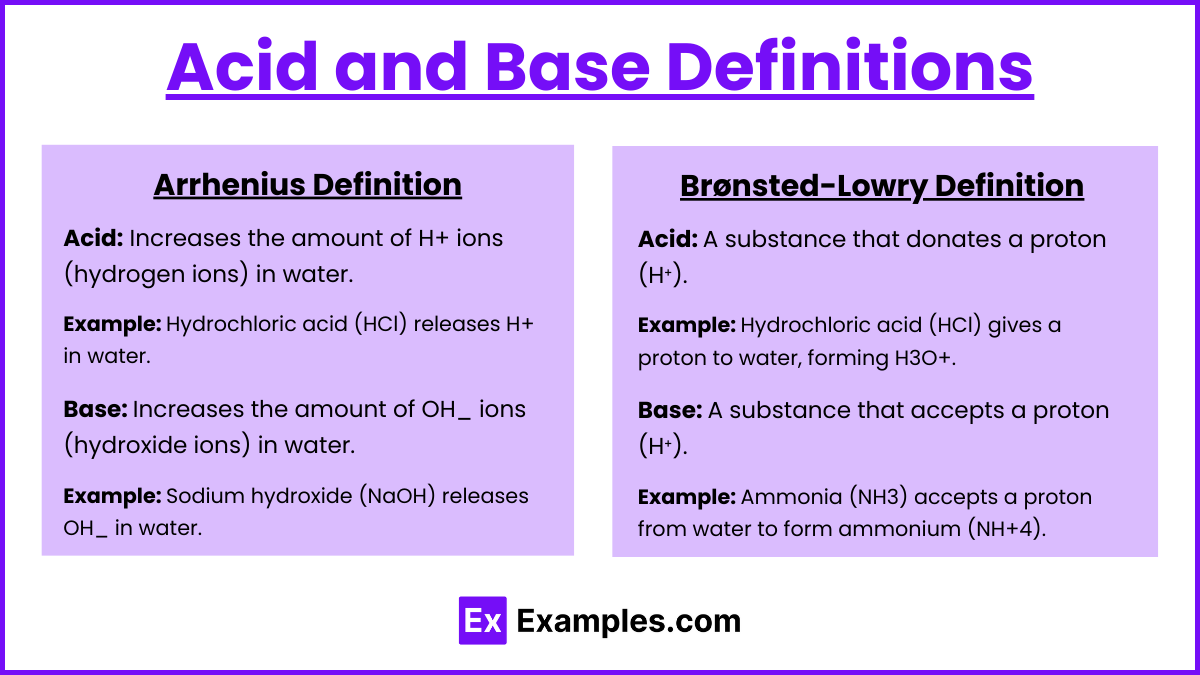
Arrhenius Definition
Acid: Increases the amount of H+ ions (hydrogen ions) in water.
Example: Hydrochloric acid (HCl) releases H+ in water.
Base: Increases the amount of OH_ ions (hydroxide ions) in water.
Example: Sodium hydroxide (NaOH) releases OH_ in water.
Brønsted-Lowry Definition
Acid: A substance that donates a proton (H⁺).
Example: Hydrochloric acid (HCl) gives a proton to water, forming H3O+..
Base: A substance that accepts a proton (H⁺).
Example: Ammonia (NH3) accepts a proton from water to form ammonium (NH+4)..
The pH and pOH Scales
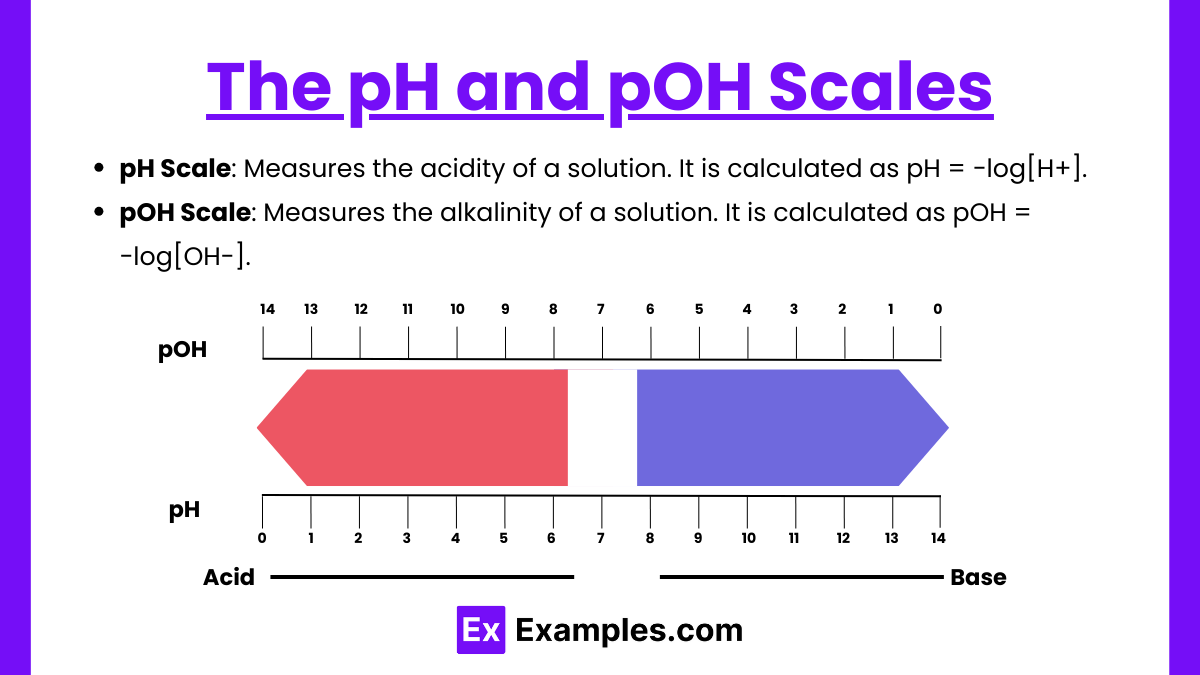
- pH Scale: Measures the acidity of a solution. It is calculated as pH= −log[H+].
- Acidic: pH < 7
- Neutral: pH = 7
- Basic: pH > 7
- pOH Scale: Measures the alkalinity of a solution. It is calculated as pOH= −log[OH–].
- pH + pOH = 14 for all aqueous solutions at 25°C.
Dissociation Constants ( Ka and Kb )
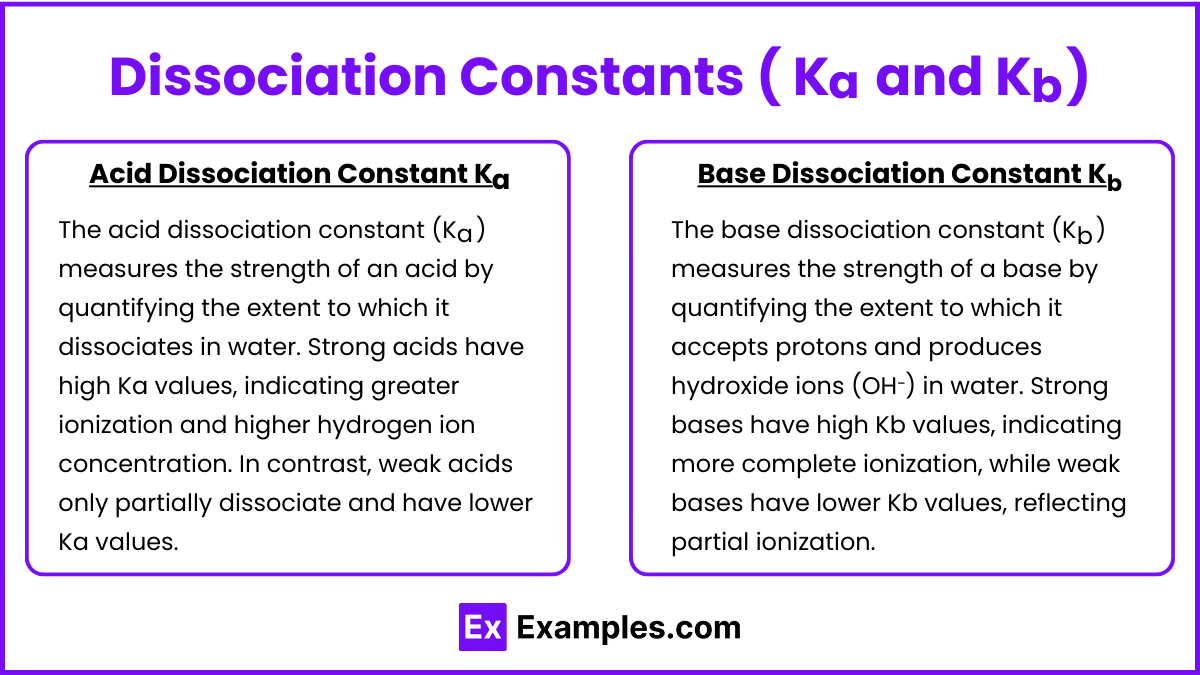
Acid Dissociation Constant Ka: Reflects the strength of an acid in water. Strong acids have high Ka values, indicating greater ionization. The acid dissociation constant (Ka) measures the strength of an acid by quantifying the extent to which it dissociates in water. Strong acids have high Ka values, indicating greater ionization and higher hydrogen ion concentration. In contrast, weak acids only partially dissociate and have lower Ka values.
Example:
For the Reaction
The expression for (K_a) is
Base Dissociation Constant Kb: Measures the strength of a base. Strong bases have high Kb values. The base dissociation constant (Kb) measures the strength of a base by quantifying the extent to which it accepts protons and produces hydroxide ions (OH⁻) in water. Strong bases have high Kb values, indicating more complete ionization, while weak bases have lower Kb values, reflecting partial ionization.
Example:
For the reaction:
The expression for
Titration and Equivalence Point
- Titration: A method used to determine the concentration of an acid or base in a solution by adding a titrant.
- Equivalence Point: The point in a titration where the amount of acid equals the amount of base.
- Strong Acid/Strong Base Titration: Equivalence point at pH 7.
- Weak Acid/Strong Base Titration: Equivalence point above pH 7.
Examples
Example 1: Dissociation of Acetic Acid in Water
Acetic acid (CH3COOH) is a weak acid that partially dissociates in water to form hydrogen ions (H+) and acetate ions (CH3COO–). The equilibrium can be represented as: CH3COOH↔H++CH3COO–
Since acetic acid is weak, only a small fraction dissociates, leading to an equilibrium state where the concentrations of the reactants and products remain constant.
Example 2: Dissociation of Ammonia in Water
Ammonia (NH3) is a weak base that reacts with water to form ammonium ions (NH4+) and hydroxide ions (OH–). The equilibrium reaction is: NH3+H2O↔NH4+ + OH_
In this equilibrium, ammonia does not completely ionize, resulting in an equilibrium between the base and its conjugate acid in water.
Example 3: Carbonic Acid and Bicarbonate Buffer System
In the blood, the carbonic acid (H2CO3) and bicarbonate (HCO3–) buffer system maintains pH balance. The equilibrium reaction is: H2CO3↔H+ +HCO3–
When excess hydrogen ions are introduced, bicarbonate ions neutralize them, and when hydroxide ions are introduced, carbonic acid releases hydrogen ions to maintain pH stability in the body.
Example 4: Hydrochloric Acid in Water
Hydrochloric acid (HCl) is a strong acid that fully dissociates in water to form hydrogen ions (H+) and chloride ions (Cl–). The reaction can be written as: HCl→H+ + Cl_
Since hydrochloric acid is strong, there is no equilibrium between the reactants and products; all HCl molecules dissociate completely in water.
Example 5: Titration of Weak Acid with Strong Base
In a titration of acetic acid (CH3COOH) with sodium hydroxide (NaOH), an equilibrium is established as the base neutralizes the weak acid.
The reaction is : CH3COOH+OH–→CH3COO– + H2O
As the titration progresses, the equilibrium shifts, and the pH changes, with the equivalence point typically above pH 7 for a weak acid-strong base titration.
Practice Questions
Question 1
Which of the following statements is true regarding the dissociation of a weak acid in water?
A) A weak acid completely dissociates into its ions.
B) The dissociation of a weak acid produces only hydroxide ions.
C) A weak acid only partially dissociates, establishing an equilibrium.
D) A weak acid cannot donate protons to a base.
Answer: C) A weak acid only partially dissociates, establishing an equilibrium.
Explanation: Weak acids do not fully dissociate in water. Instead, they establish an equilibrium between the undissociated acid and its ions. For example, acetic acid (CH3COOH) partially dissociates into hydrogen ions (H+) and acetate ions (CH3COO–). The reaction reaches a point where the rate of dissociation equals the rate of recombination, forming a dynamic equilibrium.
Question 2
What is the pH of a solution with a hydrogen ion concentration of 1×10-3 M ?
A) 3
B) 7
C) 11
D) 1
Answer: A) 3
Explanation: The pH of a solution is calculated using the formula: pH = −log[H+]
Given the hydrogen ion concentration [H+] = 1 × 10-3, the calculation is:
pH = −log(1 × 10-3) = 3
Therefore, the pH of the solution is 3, indicating an acidic solution.
Question 3
In the titration of a weak acid with a strong base, what is the pH at the equivalence point?
A) Less than 7
B) Equal to 7
C) Greater than 7
D) Exactly 0
Answer: C) Greater than 7
Explanation: In the titration of a weak acid with a strong base, the equivalence point occurs when all the weak acid has been neutralized by the strong base. At this point, the solution contains the conjugate base of the weak acid, which hydrolyzes to produce hydroxide ions (OH–), making the solution basic. As a result, the pH at the equivalence point is greater than 7.
For example, in the titration of acetic acid (CH3COOH) with sodium hydroxide (NaOH), the acetate ion (CH3COO–) is produced and reacts with water to form hydroxide ions, raising the pH above 7.
Acid/base equilibria explore the balance between acids and bases in chemical solutions, a concept crucial to understanding biochemical processes and physiological systems. It involves key principles such as pH, pKa, and the dissociation of acids and bases, alongside the behavior of buffers that resist pH changes. This topic also covers the application of the Henderson-Hasselbalch equation, titration curves, and indicators used to detect equivalence points.
Learning Objectives
In studying "Acid/Base Equilibria" for the MCAT, you should understand key concepts, including pH, pKa, and pKb, and how they relate to acid and base strength. Analyze the behavior of strong and weak acids/bases in equilibrium and apply the Henderson-Hasselbalch equation to buffer systems. Evaluate titration curves, equivalence points, and the role of indicators in acid-base reactions. Explore the application of Le Chatelier’s principle to predict equilibrium shifts. Additionally, develop skills to interpret experimental data, solve pH-related problems, and assess buffer efficiency in MCAT practice passages, focusing on how acid/base chemistry is used in biological and clinical contexts.
Acid and Base Definitions

Arrhenius Definition
Acid: Increases the amount of H+ ions (hydrogen ions) in water.
Example: Hydrochloric acid (HCl) releases H+ in water.
HCl→H++Cl−$\text{HCl} \rightarrow \text{H}^+ + \text{Cl}^-$
Base: Increases the amount of OH_ ions (hydroxide ions) in water.
Example: Sodium hydroxide (NaOH) releases OH_ in water.
NaOH→Na++OH−$\text{NaOH} \rightarrow \text{Na}^+ + \text{OH}^-$
Brønsted-Lowry Definition
Acid: A substance that donates a proton (H⁺).
Example: Hydrochloric acid (HCl) gives a proton to water, forming H3O+.
HCl+H2O→H3O++Cl−$\text{HCl} + \text{H}_2\text{O} \rightarrow \text{H}_3\text{O}^+ + \text{Cl}^-$.
Base: A substance that accepts a proton (H⁺).
Example: Ammonia (NH3) accepts a proton from water to form ammonium (NH+4).
NH3+H2O→NH4++OH−$\text{NH}_3 + \text{H}_2\text{O} \rightarrow \text{NH}_4^+ + \text{OH}^-$.
The pH and pOH Scales

pH Scale: Measures the acidity of a solution. It is calculated as pH= −log[H+].
Acidic: pH < 7
Neutral: pH = 7
Basic: pH > 7
pOH Scale: Measures the alkalinity of a solution. It is calculated as pOH= −log[OH-].
pH + pOH = 14 for all aqueous solutions at 25°C.
Dissociation Constants ( Ka and Kb )

Acid Dissociation Constant Ka: Reflects the strength of an acid in water. Strong acids have high Ka values, indicating greater ionization. The acid dissociation constant (Ka) measures the strength of an acid by quantifying the extent to which it dissociates in water. Strong acids have high Ka values, indicating greater ionization and higher hydrogen ion concentration. In contrast, weak acids only partially dissociate and have lower Ka values.
Example:
For the Reaction HA↔H++A−$\text{HA} \leftrightarrow \text{H}^+ + \text{A}^-$
The expression for (K_a) is Ka=[HA][H+][A−]$K_a = \frac{[\text{H}^+][\text{A}^-]}{[\text{HA}]}$
Base Dissociation Constant Kb: Measures the strength of a base. Strong bases have high Kb values. The base dissociation constant (Kb) measures the strength of a base by quantifying the extent to which it accepts protons and produces hydroxide ions (OH⁻) in water. Strong bases have high Kb values, indicating more complete ionization, while weak bases have lower Kb values, reflecting partial ionization.
Example:
For the reaction: B+H2O↔BH++OH−$\text{B} + \text{H}_2\text{O} \leftrightarrow \text{BH}^+ + \text{OH}^- $
The expression for Kb=[B][BH+][OH−]$K_b = \frac{[\text{BH}^+][\text{OH}^-]}{[\text{B}]}$
Titration and Equivalence Point

Titration: A method used to determine the concentration of an acid or base in a solution by adding a titrant.
Equivalence Point: The point in a titration where the amount of acid equals the amount of base.
Strong Acid/Strong Base Titration: Equivalence point at pH 7.
Weak Acid/Strong Base Titration: Equivalence point above pH 7.
Examples
Example 1: Dissociation of Acetic Acid in Water
Acetic acid (CH3COOH) is a weak acid that partially dissociates in water to form hydrogen ions (H+) and acetate ions (CH3COO-). The equilibrium can be represented as: CH3COOH↔H++CH3COO-
Since acetic acid is weak, only a small fraction dissociates, leading to an equilibrium state where the concentrations of the reactants and products remain constant.
Example 2: Dissociation of Ammonia in Water
Ammonia (NH3) is a weak base that reacts with water to form ammonium ions (NH4+) and hydroxide ions (OH-). The equilibrium reaction is: NH3+H2O↔NH4+ + OH_
In this equilibrium, ammonia does not completely ionize, resulting in an equilibrium between the base and its conjugate acid in water.
Example 3: Carbonic Acid and Bicarbonate Buffer System
In the blood, the carbonic acid (H2CO3) and bicarbonate (HCO3-) buffer system maintains pH balance. The equilibrium reaction is: H2CO3↔H+ +HCO3-
When excess hydrogen ions are introduced, bicarbonate ions neutralize them, and when hydroxide ions are introduced, carbonic acid releases hydrogen ions to maintain pH stability in the body.
Example 4: Hydrochloric Acid in Water
Hydrochloric acid (HCl) is a strong acid that fully dissociates in water to form hydrogen ions (H+) and chloride ions (Cl-). The reaction can be written as: HCl→H+ + Cl_
Since hydrochloric acid is strong, there is no equilibrium between the reactants and products; all HCl molecules dissociate completely in water.
Example 5: Titration of Weak Acid with Strong Base
In a titration of acetic acid (CH3COOH) with sodium hydroxide (NaOH), an equilibrium is established as the base neutralizes the weak acid.
The reaction is : CH3COOH+OH-→CH3COO- + H2O
As the titration progresses, the equilibrium shifts, and the pH changes, with the equivalence point typically above pH 7 for a weak acid-strong base titration.
Practice Questions
Question 1
Which of the following statements is true regarding the dissociation of a weak acid in water?
A) A weak acid completely dissociates into its ions.
B) The dissociation of a weak acid produces only hydroxide ions.
C) A weak acid only partially dissociates, establishing an equilibrium.
D) A weak acid cannot donate protons to a base.
Answer: C) A weak acid only partially dissociates, establishing an equilibrium.
Explanation: Weak acids do not fully dissociate in water. Instead, they establish an equilibrium between the undissociated acid and its ions. For example, acetic acid (CH3COOH) partially dissociates into hydrogen ions (H+) and acetate ions (CH3COO-). The reaction reaches a point where the rate of dissociation equals the rate of recombination, forming a dynamic equilibrium.
Question 2
What is the pH of a solution with a hydrogen ion concentration of 1×10-3 M ?
A) 3
B) 7
C) 11
D) 1
Answer: A) 3
Explanation: The pH of a solution is calculated using the formula: pH = −log[H+]
Given the hydrogen ion concentration [H+] = 1 × 10-3, the calculation is:
pH = −log(1 × 10-3) = 3
Therefore, the pH of the solution is 3, indicating an acidic solution.
Question 3
In the titration of a weak acid with a strong base, what is the pH at the equivalence point?
A) Less than 7
B) Equal to 7
C) Greater than 7
D) Exactly 0
Answer: C) Greater than 7
Explanation: In the titration of a weak acid with a strong base, the equivalence point occurs when all the weak acid has been neutralized by the strong base. At this point, the solution contains the conjugate base of the weak acid, which hydrolyzes to produce hydroxide ions (OH-), making the solution basic. As a result, the pH at the equivalence point is greater than 7.
For example, in the titration of acetic acid (CH3COOH) with sodium hydroxide (NaOH), the acetate ion (CH3COO-) is produced and reacts with water to form hydroxide ions, raising the pH above 7.