Aldehydes and ketones are carbonyl-containing compounds that play essential roles in organic chemistry, particularly in metabolism, synthesis, and reaction mechanisms. Both feature a carbon double-bonded to oxygen, but differ in the placement of this bond. Aldehydes have the carbonyl group at the end of a carbon chain, while ketones have it within the chain. Understanding their structures, properties, and reactivity is critical for grasping broader biochemical processes, making them a key topic for the MCAT exam.
Learning Objectives
In studying “Aldehydes and Ketones” for the MCAT involves understanding their structure, naming, and functional group placement. Focus on key reactions like nucleophilic addition, oxidation, and reduction, including the use of reagents like PCC and NaBH₄. Emphasize enolate formation, tautomerization, and aldol condensation, which are crucial for linking to metabolic pathways. Additionally, explore how aldehydes and ketones play roles in biological processes like glycolysis and the citric acid cycle, highlighting their importance in energy metabolism and biomolecule synthesis.
1. Introduction to Aldehydes and Ketones
- Aldehydes and ketones are organic compounds with a carbonyl group. The carbonyl group in aldehydes is terminal (at the end of the carbon chain), while in ketones, it is internal (within the chain).
- They are polar molecules, and their reactions often involve nucleophilic attack at the carbonyl carbon, making them central to many biological and chemical processes.
2. Structure and Properties
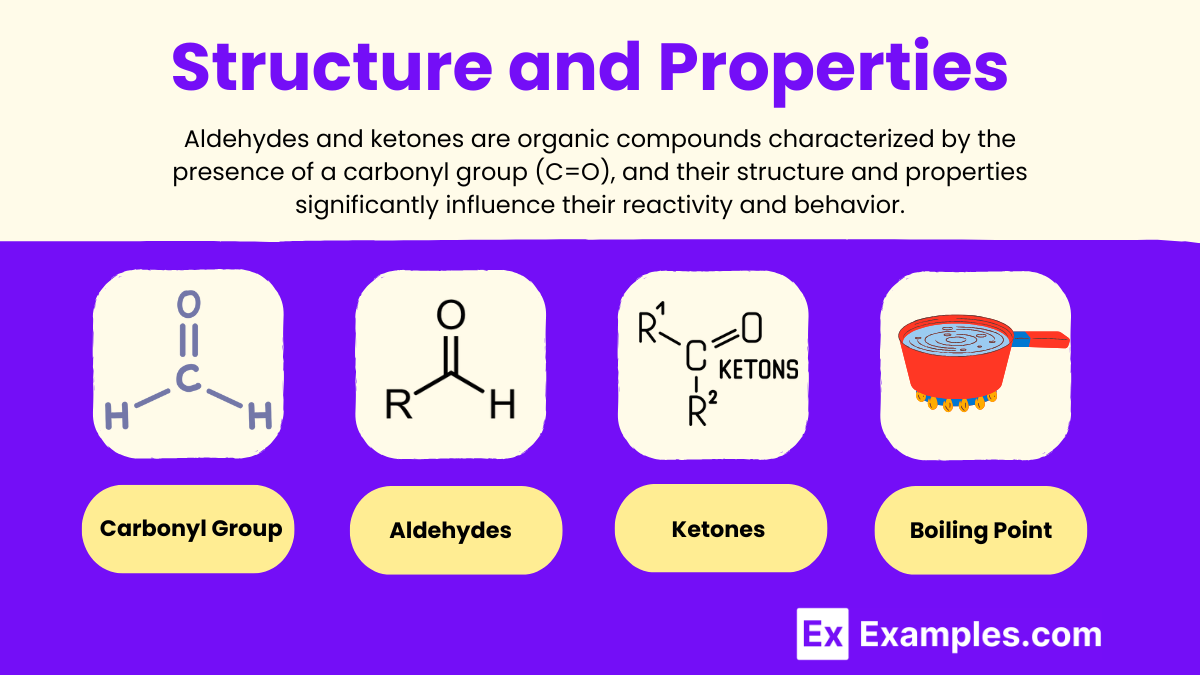
- Carbonyl Group: Both aldehydes and ketones possess a carbon-oxygen double bond, known as the carbonyl group, which is highly reactive due to its polar nature.
- Aldehydes: Represented as R-CHO, where ‘R’ is any alkyl or aryl group. The hydrogen atom attached to the carbonyl carbon makes aldehydes more prone to oxidation.
- Ketones: Denoted as R-CO-R’, where ‘R’ and ‘R’’ are alkyl or aryl groups. Ketones are less reactive than aldehydes but more stable.
- Boiling Point: Higher than alkanes but lower than alcohols due to the lack of hydrogen bonding. They can form hydrogen bonds with water, making lower aldehydes and ketones soluble in water.
3. Biological Relevance of Aldehydes and Ketones
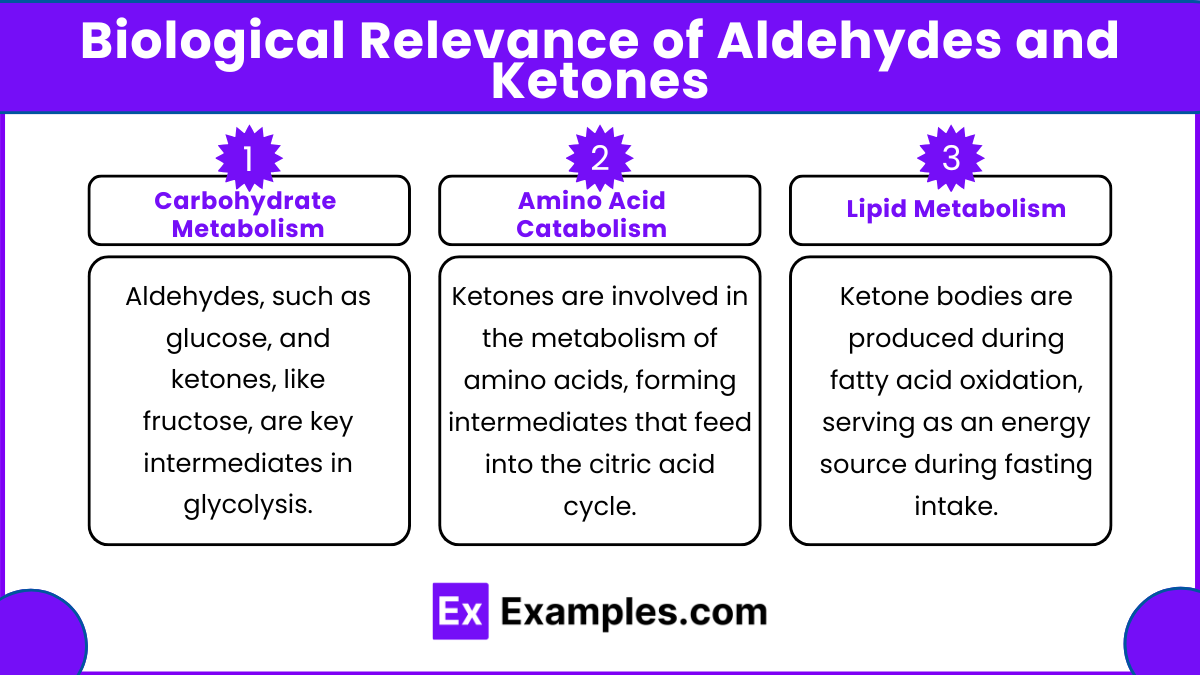
- Carbohydrate Metabolism: Aldehydes, such as glucose, and ketones, like fructose, are key intermediates in glycolysis.
- Amino Acid Catabolism: Ketones are involved in the metabolism of amino acids, forming intermediates that feed into the citric acid cycle.
- Lipid Metabolism: Ketone bodies (e.g., acetoacetate, β-hydroxybutyrate) are produced during fatty acid oxidation, serving as an energy source during fasting or low-carbohydrate intake.
Examples
Example 1. Flavoring Agents in Food Industry
Aldehydes, like vanillin (from vanilla beans) and cinnamaldehyde (from cinnamon), are widely used in the food industry as flavoring agents. These aldehydes add specific aromas to various foods, enhancing the sensory experience. Ketones, such as diacetyl, impart a buttery flavor and are used in products like popcorn, baked goods, and margarine. The reactivity of aldehydes and ketones allows for the formation of complex flavor molecules when combined with other ingredients.
Example 2. Chemical Synthesis of Pharmaceuticals
Aldehydes and ketones are vital intermediates in the synthesis of many drugs. For instance, acetone (a ketone) is used in the synthesis of cortisone, a steroidal drug, while formaldehyde (an aldehyde) is used in the preparation of methenamine, an antiseptic. These compounds undergo various reactions, such as nucleophilic addition, to form new structures, showcasing their importance in medicinal chemistry and drug development.
Example 3. Biochemical Pathways in the Human Body
In human metabolism, aldehydes and ketones play key roles. Glucose, an aldehyde, undergoes glycolysis to produce pyruvate, an essential intermediate in cellular respiration. Similarly, ketone bodies (e.g., acetoacetate and β-hydroxybutyrate), formed during fatty acid breakdown, provide an alternative energy source during fasting. These metabolic pathways illustrate how aldehydes and ketones are critical for energy production and utilization in the body.
Example 4. Polymer Production in Industrial Chemistry
Aldehydes, such as formaldehyde, are used in the production of polymers like phenol-formaldehyde resin (Bakelite), a durable and heat-resistant plastic. Ketones, such as methyl ethyl ketone (MEK), serve as solvents in polymer production, aiding in the creation of synthetic materials like polyvinyl chloride (PVC). The reactivity of aldehydes and ketones in polymerization processes underlines their significance in material science.
Example 5. Analytical Chemistry and Testing
Aldehydes and ketones are frequently used as analytical reagents. Formaldehyde reacts with proteins to create cross-linking in tissue samples, preserving them for histological studies. Similarly, 2,4-Dinitrophenylhydrazine (DNPH) is used to detect aldehydes and ketones in solutions by forming a yellow or orange precipitate, which helps in identifying and quantifying these compounds in laboratory settings.
Practice Questions
Question 1
Which of the following functional groups is present in both aldehydes and ketones?
a) Hydroxyl group
b) Carbonyl group
c) Amino group
d) Carboxyl group
Answer: b) Carbonyl group
Explanation: Both aldehydes and ketones contain a carbonyl group (C=O), which is the defining feature of these functional groups. In aldehydes, the carbonyl carbon is bonded to at least one hydrogen atom and one carbon atom, while in ketones, the carbonyl carbon is bonded to two carbon atoms. The carbonyl group is highly reactive and plays a crucial role in the reactivity of both aldehydes and ketones. Other options listed do not define aldehydes or ketones specifically.
Question 2
Which reagent is commonly used to distinguish between aldehydes and ketones?
a) Sodium borohydride (NaBH₄)
b) Tollen’s reagent
c) Grignard reagent
d) Acetic acid
Answer: b) Tollen’s reagent
Explanation: Tollen’s reagent, which contains ammoniacal silver nitrate (Ag(NH₃)₂⁺), is used to distinguish between aldehydes and ketones. Aldehydes reduce Tollen’s reagent, forming a silver mirror on the test tube, while ketones do not react because they lack the hydrogen atom needed for oxidation. This selective reaction is due to the fact that aldehydes are more readily oxidized than ketones, making Tollen’s reagent an effective tool for identifying aldehydes specifically.
Question 3
What is the primary product formed when an aldehyde undergoes oxidation?
a) Alcohol
b) Ester
c) Carboxylic acid
d) Ketone
Answer: c) Carboxylic acid
Explanation: When an aldehyde is oxidized, it forms a carboxylic acid as the primary product. The carbonyl carbon in the aldehyde is further oxidized by gaining an oxygen atom, converting it into a carboxyl group (-COOH). This reaction typically occurs with mild oxidizing agents like potassium permanganate (KMnO₄) or chromium-based reagents. Ketones, in contrast, do not readily undergo further oxidation under similar conditions due to the absence of a hydrogen atom directly attached to the carbonyl carbon.