Amino acids and proteins

- Notes
Amino acids and proteins are fundamental to biological processes, acting as building blocks for cell structure and function. On the MCAT, you’ll explore amino acid classifications, peptide bond formation, and protein structures. Understanding enzyme kinetics, protein folding, and denaturation is crucial, as these concepts are key to mastering biochemistry and molecular biology sections of the exam.
Learning Objectives
In studying “Amino Acids and Proteins” for the MCAT, you should learn to identify the structure, properties, and classification of amino acids, including their side chains and behavior in different pH environments. Understand how amino acids link to form peptides and the four levels of protein structure (primary, secondary, tertiary, and quaternary). Analyze protein folding, stability, and the role of non-covalent interactions in maintaining protein shape. Evaluate enzyme function, including catalysis, substrate specificity, and factors affecting enzyme activity. Additionally, understand the significance of proteins in biological processes such as cell signaling, transport, and metabolism.
Amino Acids Structure and Properties
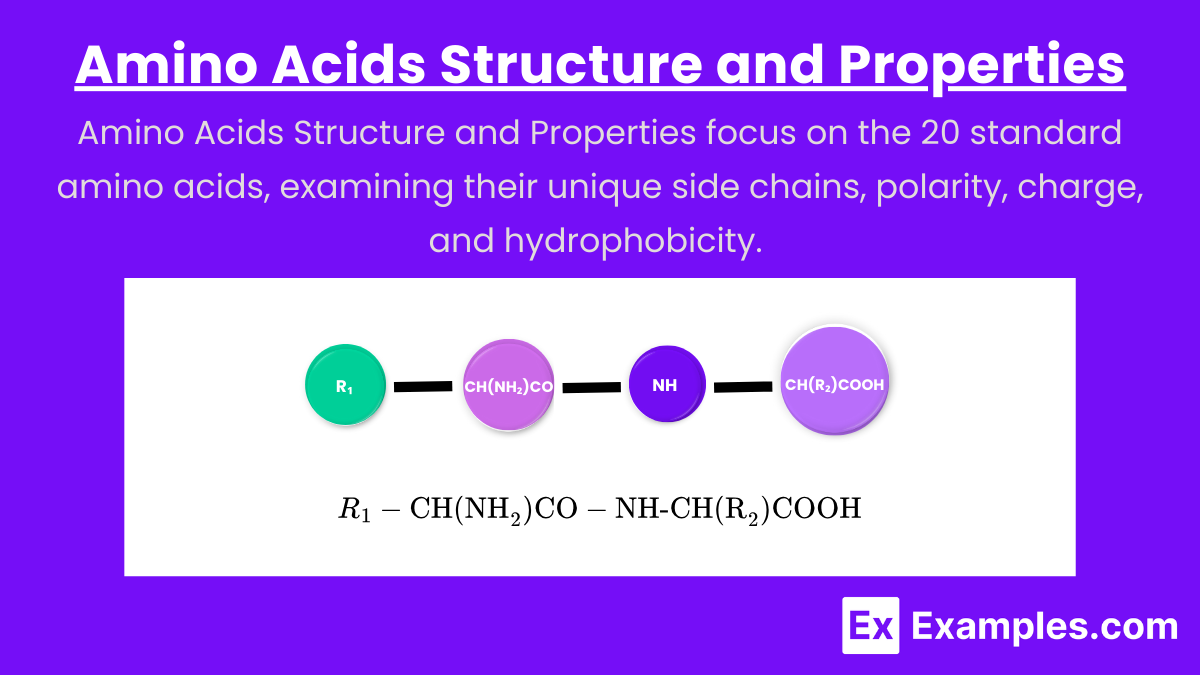
- Basic Structure: Amino acids consist of a central carbon atom (α-carbon) attached to an amino group (-NH₂), a carboxyl group (-COOH), a hydrogen atom, and a variable side chain (R-group).
- Classification:
- Based on R-group Properties: Nonpolar, polar, acidic, and basic.
- Essential vs. Non-essential: Essential amino acids must be obtained from the diet.
- Acid-Base Chemistry: Amino acids are zwitterions, meaning they can exist with both positive and negative charges. They can act as acids or bases depending on the pH.
- Isoelectric Point (pI): The pH at which an amino acid carries no net charge. Acidic amino acids have lower pI values, while basic ones have higher pI values.
Peptide Bonds and Protein Structure
- Formation of Peptide Bonds: Amino acids link together via peptide bonds formed through a dehydration reaction between the amino group of one amino acid and the carboxyl group of another.
- Levels of Protein Structure:
- Primary Structure: Linear sequence of amino acids in a polypeptide chain.
- Secondary Structure: Local folding patterns, including alpha helices and beta-pleated sheets, stabilized by hydrogen bonding.
- Tertiary Structure: Three-dimensional folding of a polypeptide, stabilized by interactions among R-groups (hydrophobic interactions, disulfide bridges, hydrogen bonds, and ionic interactions).
- Quaternary Structure: Assembly of multiple polypeptide chains into a functional protein (e.g., hemoglobin).
Protein Function and Enzymes
Protein Function and Enzymes explore how proteins serve diverse roles such as catalysis, transport, and signaling. Enzymes, a key protein type, accelerate biochemical reactions by lowering activation energy, exhibiting specificity for substrates, and playing critical roles in regulating metabolic pathways.
- Functions: Proteins have diverse roles, including structural support (collagen), transport (hemoglobin), signaling (hormones), and catalysis (enzymes).
- Enzyme Kinetics:
- Active Site: The region where substrate binds and catalysis occurs.
- Mechanism of Action: Enzymes lower activation energy, facilitating faster reactions. They do not alter the equilibrium.
- Enzyme Inhibition: Types include competitive, non-competitive, and uncompetitive inhibition.
- Enzyme Regulation: Mechanisms include allosteric control, covalent modification (e.g., phosphorylation), and feedback inhibition.
Protein Synthesis and Post-Translational Modifications
- Synthesis Process: Involves transcription (DNA to RNA) and translation (RNA to protein). Ribosomes facilitate translation by reading mRNA and assembling amino acids into polypeptide chains.
- Post-Translational Modifications: Include phosphorylation, glycosylation, and ubiquitination, which affect protein function, localization, and degradation.
Examples
Example 1: Glycine
As the simplest amino acid, glycine has a single hydrogen atom as its R-group, making it flexible and frequently found in protein structures that require tight turns. Glycine is non-polar and plays a role in collagen, where it helps maintain structural integrity.
Example 2: Hemoglobin
This is a quaternary protein consisting of four polypeptide subunits, each capable of binding one oxygen molecule. Found in red blood cells, hemoglobin’s main role is oxygen transport. Its structure allows for cooperative binding, making it efficient at picking up oxygen in the lungs and releasing it in tissues.
Example 3: Cysteine
Known for its thiol (-SH) group, cysteine can form disulfide bonds with another cysteine. These bonds contribute to the stability of protein structures by linking different parts of a protein or even different polypeptides, as seen in keratin, the protein responsible for hair and nail strength.
Example 4: Myosin
This motor protein is essential in muscle contraction. Myosin interacts with actin filaments in muscle fibers, using ATP to generate force and facilitate movement. The interaction between myosin and actin is fundamental to both voluntary and involuntary muscle actions.
Example 5: Insulin
As a peptide hormone, insulin is crucial in regulating blood glucose levels. Produced by the pancreas, it consists of two polypeptide chains linked by disulfide bonds. Insulin enables cells to take up glucose from the blood, thus playing a critical role in energy metabolism and maintaining homeostasis.
Practice Questions
Question 1:
Which of the following amino acids contains a sulfur atom in its side chain?
A) Alanine
B) Glycine
C) Methionine
D) Lysine
Answer: C) Methionine
Explanation: Methionine contains a sulfur atom in its side chain, making it one of the two sulfur-containing amino acids, along with cysteine. Sulfur is part of methionine’s side chain in the form of a thioether group. Alanine and glycine are simple amino acids with non-polar side chains and do not contain sulfur, while lysine has a positively charged, basic side chain with no sulfur.
Question 2:
Which level of protein structure is primarily stabilized by hydrogen bonds between backbone carbonyl and amino groups?
A) Primary
B) Secondary
C) Tertiary
D) Quaternary
Answer: B) Secondary
Explanation: The secondary structure of proteins includes alpha helices and beta sheets, which are stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amine hydrogen of another along the protein backbone. The primary structure is the amino acid sequence itself, and tertiary and quaternary structures are stabilized by various interactions, including hydrogen bonds, but also hydrophobic interactions, ionic bonds, and disulfide bridges.
Question 3:
If an enzyme has a high Km for a substrate, what does this indicate about the enzyme’s affinity for that substrate?
A) The enzyme has a high affinity for the substrate.
B) The enzyme has a low affinity for the substrate.
C) The enzyme is denatured.
D) The enzyme’s activity is unaffected by substrate concentration.
Answer: B) The enzyme has a low affinity for the substrate.
Explanation: Km represents the substrate concentration at which the reaction rate is half of its maximum (Vmax). A high Km indicates that a relatively high concentration of substrate is needed to reach half of Vmax, implying that the enzyme has a low affinity for the substrate. Conversely, a low Km value would indicate a high affinity, meaning that the enzyme can reach half of its maximum rate at a lower substrate concentration.
Amino acids and proteins are fundamental to biological processes, acting as building blocks for cell structure and function. On the MCAT, you’ll explore amino acid classifications, peptide bond formation, and protein structures. Understanding enzyme kinetics, protein folding, and denaturation is crucial, as these concepts are key to mastering biochemistry and molecular biology sections of the exam.
Learning Objectives
In studying "Amino Acids and Proteins" for the MCAT, you should learn to identify the structure, properties, and classification of amino acids, including their side chains and behavior in different pH environments. Understand how amino acids link to form peptides and the four levels of protein structure (primary, secondary, tertiary, and quaternary). Analyze protein folding, stability, and the role of non-covalent interactions in maintaining protein shape. Evaluate enzyme function, including catalysis, substrate specificity, and factors affecting enzyme activity. Additionally, understand the significance of proteins in biological processes such as cell signaling, transport, and metabolism.
Amino Acids Structure and Properties
Basic Structure: Amino acids consist of a central carbon atom (α-carbon) attached to an amino group (-NH₂), a carboxyl group (-COOH), a hydrogen atom, and a variable side chain (R-group).
Classification:
Based on R-group Properties: Nonpolar, polar, acidic, and basic.
Essential vs. Non-essential: Essential amino acids must be obtained from the diet.
Acid-Base Chemistry: Amino acids are zwitterions, meaning they can exist with both positive and negative charges. They can act as acids or bases depending on the pH.
Isoelectric Point (pI): The pH at which an amino acid carries no net charge. Acidic amino acids have lower pI values, while basic ones have higher pI values.
Peptide Bonds and Protein Structure
Formation of Peptide Bonds: Amino acids link together via peptide bonds formed through a dehydration reaction between the amino group of one amino acid and the carboxyl group of another.
Levels of Protein Structure:
Primary Structure: Linear sequence of amino acids in a polypeptide chain.
Secondary Structure: Local folding patterns, including alpha helices and beta-pleated sheets, stabilized by hydrogen bonding.
Tertiary Structure: Three-dimensional folding of a polypeptide, stabilized by interactions among R-groups (hydrophobic interactions, disulfide bridges, hydrogen bonds, and ionic interactions).
Quaternary Structure: Assembly of multiple polypeptide chains into a functional protein (e.g., hemoglobin).
Protein Function and Enzymes
Protein Function and Enzymes explore how proteins serve diverse roles such as catalysis, transport, and signaling. Enzymes, a key protein type, accelerate biochemical reactions by lowering activation energy, exhibiting specificity for substrates, and playing critical roles in regulating metabolic pathways.
Functions: Proteins have diverse roles, including structural support (collagen), transport (hemoglobin), signaling (hormones), and catalysis (enzymes).
Enzyme Kinetics:
Active Site: The region where substrate binds and catalysis occurs.
Mechanism of Action: Enzymes lower activation energy, facilitating faster reactions. They do not alter the equilibrium.
Enzyme Inhibition: Types include competitive, non-competitive, and uncompetitive inhibition.
Enzyme Regulation: Mechanisms include allosteric control, covalent modification (e.g., phosphorylation), and feedback inhibition.
Protein Synthesis and Post-Translational Modifications
Synthesis Process: Involves transcription (DNA to RNA) and translation (RNA to protein). Ribosomes facilitate translation by reading mRNA and assembling amino acids into polypeptide chains.
Post-Translational Modifications: Include phosphorylation, glycosylation, and ubiquitination, which affect protein function, localization, and degradation.
Examples
Example 1: Glycine
As the simplest amino acid, glycine has a single hydrogen atom as its R-group, making it flexible and frequently found in protein structures that require tight turns. Glycine is non-polar and plays a role in collagen, where it helps maintain structural integrity.
Example 2: Hemoglobin
This is a quaternary protein consisting of four polypeptide subunits, each capable of binding one oxygen molecule. Found in red blood cells, hemoglobin’s main role is oxygen transport. Its structure allows for cooperative binding, making it efficient at picking up oxygen in the lungs and releasing it in tissues.
Example 3: Cysteine
Known for its thiol (-SH) group, cysteine can form disulfide bonds with another cysteine. These bonds contribute to the stability of protein structures by linking different parts of a protein or even different polypeptides, as seen in keratin, the protein responsible for hair and nail strength.
Example 4: Myosin
This motor protein is essential in muscle contraction. Myosin interacts with actin filaments in muscle fibers, using ATP to generate force and facilitate movement. The interaction between myosin and actin is fundamental to both voluntary and involuntary muscle actions.
Example 5: Insulin
As a peptide hormone, insulin is crucial in regulating blood glucose levels. Produced by the pancreas, it consists of two polypeptide chains linked by disulfide bonds. Insulin enables cells to take up glucose from the blood, thus playing a critical role in energy metabolism and maintaining homeostasis.
Practice Questions
Question 1:
Which of the following amino acids contains a sulfur atom in its side chain?
A) Alanine
B) Glycine
C) Methionine
D) Lysine
Answer: C) Methionine
Explanation: Methionine contains a sulfur atom in its side chain, making it one of the two sulfur-containing amino acids, along with cysteine. Sulfur is part of methionine’s side chain in the form of a thioether group. Alanine and glycine are simple amino acids with non-polar side chains and do not contain sulfur, while lysine has a positively charged, basic side chain with no sulfur.
Question 2:
Which level of protein structure is primarily stabilized by hydrogen bonds between backbone carbonyl and amino groups?
A) Primary
B) Secondary
C) Tertiary
D) Quaternary
Answer: B) Secondary
Explanation: The secondary structure of proteins includes alpha helices and beta sheets, which are stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amine hydrogen of another along the protein backbone. The primary structure is the amino acid sequence itself, and tertiary and quaternary structures are stabilized by various interactions, including hydrogen bonds, but also hydrophobic interactions, ionic bonds, and disulfide bridges.
Question 3:
If an enzyme has a high Km for a substrate, what does this indicate about the enzyme’s affinity for that substrate?
A) The enzyme has a high affinity for the substrate.
B) The enzyme has a low affinity for the substrate.
C) The enzyme is denatured.
D) The enzyme’s activity is unaffected by substrate concentration.
Answer: B) The enzyme has a low affinity for the substrate.
Explanation: Km represents the substrate concentration at which the reaction rate is half of its maximum (Vmax). A high Km indicates that a relatively high concentration of substrate is needed to reach half of Vmax, implying that the enzyme has a low affinity for the substrate. Conversely, a low Km value would indicate a high affinity, meaning that the enzyme can reach half of its maximum rate at a lower substrate concentration.

