Amino acids, Peptides, Proteins

- Notes
Amino acids are the building blocks of proteins, which are essential for virtually all biological processes. Proteins play structural, enzymatic, signaling, and transport roles in the body. Understanding amino acid properties, peptide bond formation, and protein structure is crucial for predicting how proteins function in different biological contexts. Mastering this topic will strengthen your ability to analyze enzyme mechanisms, protein folding, and metabolic pathways for the MCAT.
Learning Objectives
In studying “Amino Acids, Peptides, and Proteins” for the MCAT, you should understand the structures and properties of amino acids, including polarity, charge, and side chain behavior. Analyze how peptide bonds form and how protein structure is organized into primary, secondary, tertiary, and quaternary levels. Evaluate the role of proteins in biological systems, including enzymes, hormones, and structural proteins. Explore key topics like denaturation, folding, and post-translational modifications. Additionally, learn to interpret experimental data related to protein function and stability, applying this understanding to MCAT practice passages focused on molecular biology and biochemistry scenarios.
What are Amino Acids?
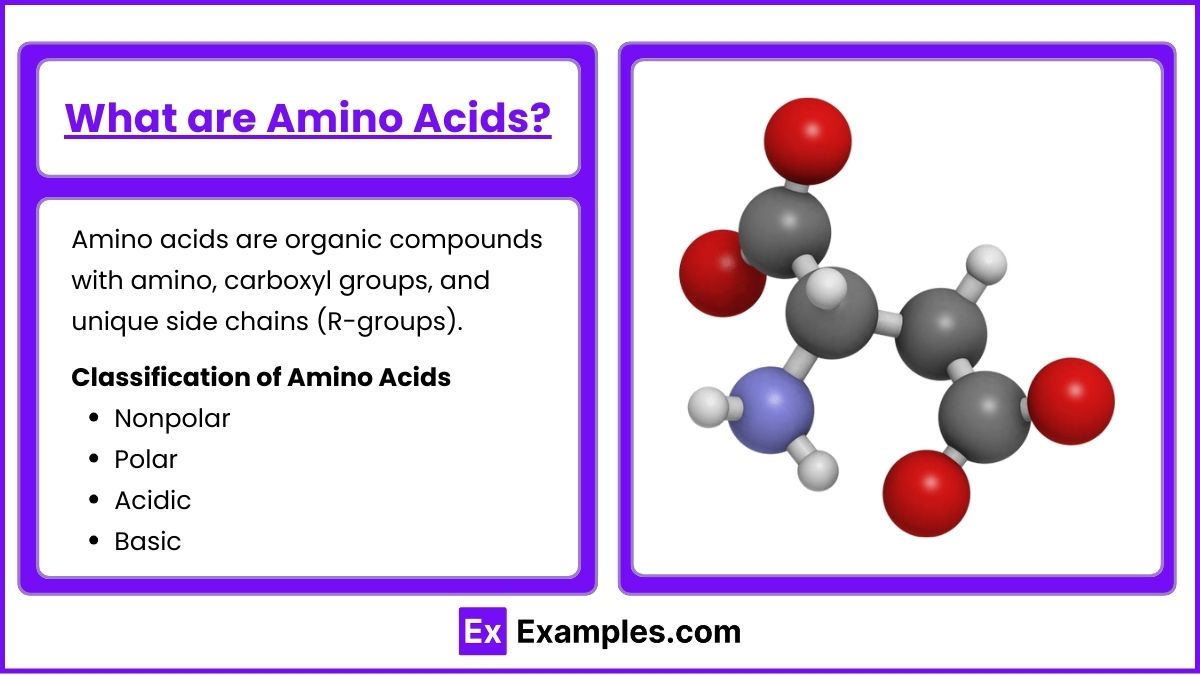
Amino acids are organic compounds that contain both an amino group ( $ \text{−NH}_2 $ ) and a carboxyl group ( $ \text{−COOH} $ ), as well as a side chain (R-group) that is unique to each amino acid. In total, there are 20 standard amino acids used to build proteins. These amino acids are classified into groups based on the characteristics of their side chains:
- Nonpolar: Hydrophobic amino acids with nonpolar side chains (e.g., glycine, alanine).
- Polar: Hydrophilic amino acids with polar side chains (e.g., serine, threonine).
- Acidic: Amino acids with carboxyl group side chains that donate protons (e.g., aspartic acid, glutamic acid).
- Basic: Amino acids with amino group side chains that accept protons (e.g., lysine, arginine).
Peptide Bond Formation
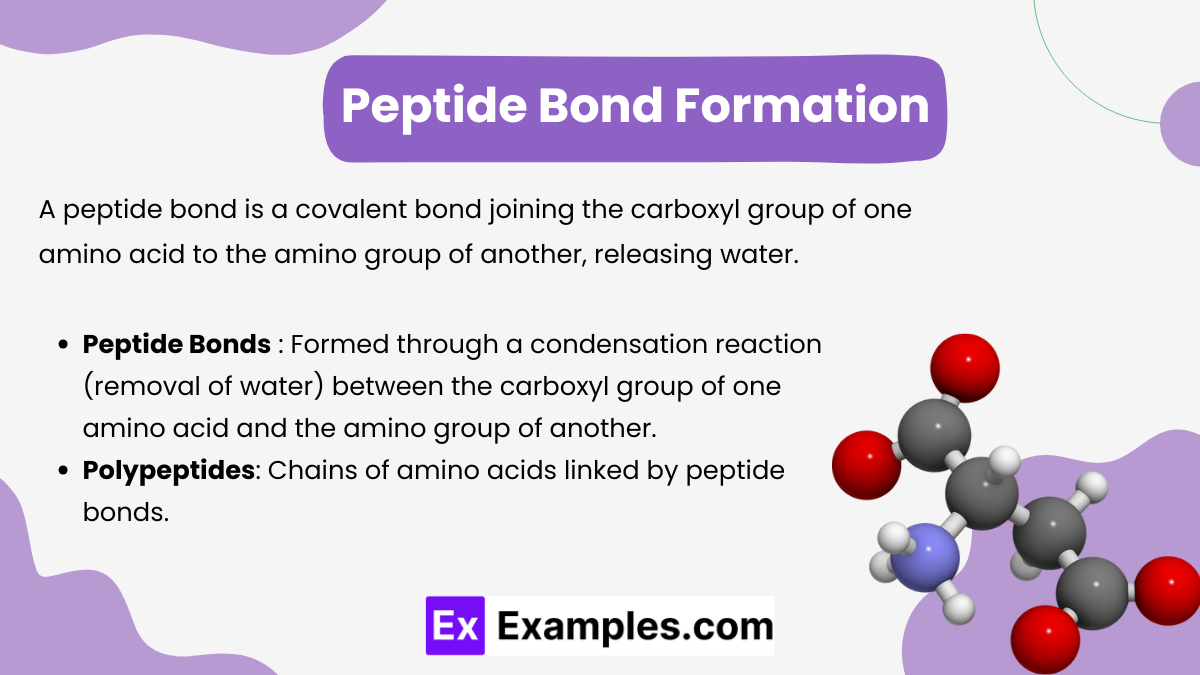
A peptide bond is a covalent bond joining the carboxyl group of one amino acid to the amino group of another, releasing water. Peptides are formed by linking amino acids together via peptide bonds. A peptide bond is a covalent bond formed between the carboxyl group of one amino acid and the amino group of another, releasing a molecule of water in the process (dehydration synthesis). The sequence of amino acids in a peptide or protein is called its primary structure.
- Peptide Bonds:
- Formed through a condensation reaction (removal of water) between the carboxyl group of one amino acid and the amino group of another.
- Peptide bonds are planar and have partial double-bond character, restricting rotation.
- Polypeptides: Chains of amino acids linked by peptide bonds.
- Oligopeptides: Short chains (few amino acids).
- Proteins: Long polypeptide chains with specific 3D structures.
Protein Structure
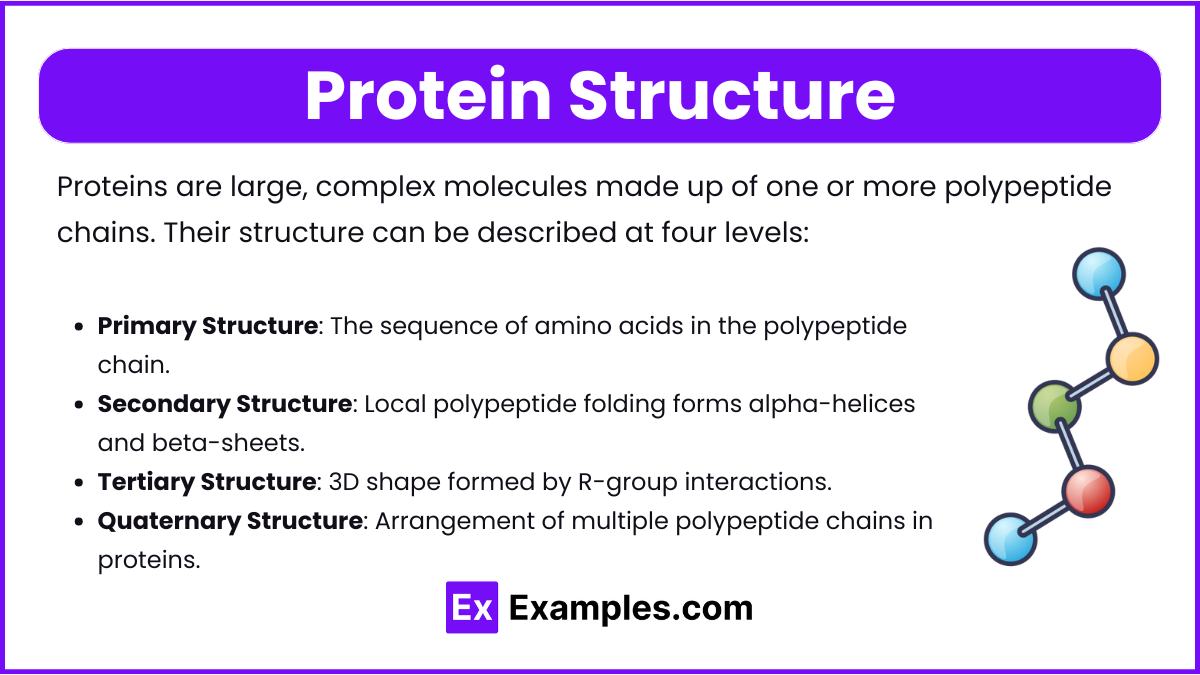
Proteins are large, complex molecules made up of one or more polypeptide chains. Their structure can be described at four levels:
- Primary Structure: The sequence of amino acids in the polypeptide chain.
- Secondary Structure: Local folding of the polypeptide chain into structures like alpha-helices and beta-sheets, stabilized by hydrogen bonds.
- Tertiary Structure: The overall 3D shape of the protein, determined by interactions between R-groups (e.g., hydrophobic interactions, disulfide bonds).
- Quaternary Structure: The arrangement of multiple polypeptide chains (subunits) in a protein, such as in hemoglobin.
Protein Denaturation
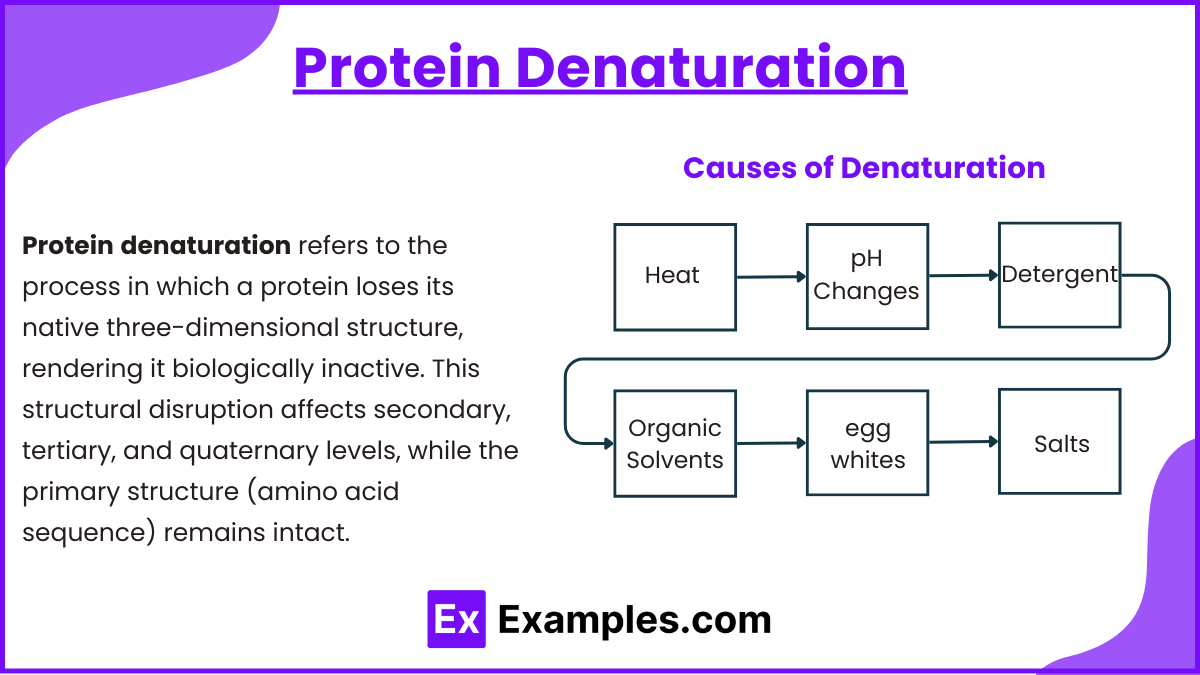
Protein denaturation refers to the process in which a protein loses its native three-dimensional structure, rendering it biologically inactive. This structural disruption affects secondary, tertiary, and quaternary levels, while the primary structure (amino acid sequence) remains intact.
Causes of Denaturation:
- Heat: Increases kinetic energy, disrupting hydrogen bonds and hydrophobic interactions (e.g., cooking egg whites).
- pH Changes: Alters the charge distribution on amino acids, disrupting ionic bonds (e.g., stomach acid denatures ingested proteins).
- Detergents: Disrupt hydrophobic interactions. Reducing Agents: Break disulfide bonds (e.g., β-mercaptoethanol).
- Organic Solvents: Disrupt hydrogen bonding (e.g., alcohols).
- Egg Whites: Shearing forces disrupt protein structure.
- Salts: High salt concentrations disrupt ionic interactions and solubility (e.g., salting out proteins).
Examples
Example 1: Glycine ( $ \text{NH}_2\text{CH}_2\text{COOH} $ )
Glycine is the simplest amino acid with a hydrogen atom as its R-group. It is nonpolar and small, allowing flexibility in proteins. Glycine is important in collagen and often found in tight turns of protein structures.
Example 2: Glutamic Acid ( $ \text{HOOC-(CH}_2\text{)}_2\text{CH(NH}_2\text{)COOH} $ )
Glutamic acid is an acidic amino acid, meaning it has a carboxyl group that can donate a proton, making it negatively charged at physiological pH. It plays a key role in neurotransmission and metabolism.
Example 3: Lysine ( $ \text{H}_2\text{N-(CH}_2\text{)}_4\text{CH(NH}_2\text{)COOH} $ )
Lysine is a basic amino acid with a long hydrocarbon chain and an amino group that accepts a proton, making it positively charged. Lysine is essential in protein interactions, particularly in histones that package DNA.
Example 4: Hemoglobin
Hemoglobin is a protein with quaternary structure composed of four polypeptide subunits. It binds oxygen in the blood and transports it throughout the body. The structure of hemoglobin allows for cooperative binding, meaning oxygen binding increases its affinity for more oxygen.
Example 5: Insulin
Insulin is a peptide hormone with two polypeptide chains connected by disulfide bonds. It regulates blood sugar levels by facilitating the uptake of glucose into cells. Insulin’s function depends on its precise 3D structure.
Practice Questions
Question 1
Which of the following amino acids has a basic side chain?
A) Aspartic acid
B) Serine
C) Lysine
D) Leucine
Answer: C) Lysine
Explanation: Lysine has a positively charged amino group in its side chain, making it basic. Aspartic acid is acidic, serine is polar, and leucine is nonpolar.
Question 2:
What type of bond connects amino acids in a protein’s primary structure?
A) Hydrogen bond
B) Disulfide bond
C) Peptide bond
D) Ionic bond
Answer: C) Peptide bond
Explanation: Peptide bonds form between the carboxyl group of one amino acid and the amino group of another, linking them into a polypeptide chain. Hydrogen bonds and disulfide bonds stabilize higher levels of protein structure, while ionic bonds may stabilize tertiary or quaternary structures.
Question 3
What is the primary structure of a protein?
A) The sequence of amino acids
B) The local folding into alpha-helices and beta-sheets
C) The overall 3D shape of the protein
D) The arrangement of multiple polypeptide chains
Answer: A) The sequence of amino acids
Explanation: The primary structure of a protein is the linear sequence of amino acids that determines the protein’s overall structure and function. Secondary, tertiary, and quaternary structures describe higher levels of folding and organization.
Amino acids are the building blocks of proteins, which are essential for virtually all biological processes. Proteins play structural, enzymatic, signaling, and transport roles in the body. Understanding amino acid properties, peptide bond formation, and protein structure is crucial for predicting how proteins function in different biological contexts. Mastering this topic will strengthen your ability to analyze enzyme mechanisms, protein folding, and metabolic pathways for the MCAT.
Learning Objectives
In studying "Amino Acids, Peptides, and Proteins" for the MCAT, you should understand the structures and properties of amino acids, including polarity, charge, and side chain behavior. Analyze how peptide bonds form and how protein structure is organized into primary, secondary, tertiary, and quaternary levels. Evaluate the role of proteins in biological systems, including enzymes, hormones, and structural proteins. Explore key topics like denaturation, folding, and post-translational modifications. Additionally, learn to interpret experimental data related to protein function and stability, applying this understanding to MCAT practice passages focused on molecular biology and biochemistry scenarios.
What are Amino Acids?

Amino acids are organic compounds that contain both an amino group ( −NH2$ \text{−NH}_2 $ ) and a carboxyl group ( −COOH$ \text{−COOH} $ ), as well as a side chain (R-group) that is unique to each amino acid. In total, there are 20 standard amino acids used to build proteins. These amino acids are classified into groups based on the characteristics of their side chains:
Nonpolar: Hydrophobic amino acids with nonpolar side chains (e.g., glycine, alanine).
Polar: Hydrophilic amino acids with polar side chains (e.g., serine, threonine).
Acidic: Amino acids with carboxyl group side chains that donate protons (e.g., aspartic acid, glutamic acid).
Basic: Amino acids with amino group side chains that accept protons (e.g., lysine, arginine).
Peptide Bond Formation

A peptide bond is a covalent bond joining the carboxyl group of one amino acid to the amino group of another, releasing water. Peptides are formed by linking amino acids together via peptide bonds. A peptide bond is a covalent bond formed between the carboxyl group of one amino acid and the amino group of another, releasing a molecule of water in the process (dehydration synthesis). The sequence of amino acids in a peptide or protein is called its primary structure.
Peptide Bonds:
Formed through a condensation reaction (removal of water) between the carboxyl group of one amino acid and the amino group of another.
Peptide bonds are planar and have partial double-bond character, restricting rotation.
Polypeptides: Chains of amino acids linked by peptide bonds.
Oligopeptides: Short chains (few amino acids).
Proteins: Long polypeptide chains with specific 3D structures.
Protein Structure

Proteins are large, complex molecules made up of one or more polypeptide chains. Their structure can be described at four levels:
Primary Structure: The sequence of amino acids in the polypeptide chain.
Secondary Structure: Local folding of the polypeptide chain into structures like alpha-helices and beta-sheets, stabilized by hydrogen bonds.
Tertiary Structure: The overall 3D shape of the protein, determined by interactions between R-groups (e.g., hydrophobic interactions, disulfide bonds).
Quaternary Structure: The arrangement of multiple polypeptide chains (subunits) in a protein, such as in hemoglobin.
Protein Denaturation

Protein denaturation refers to the process in which a protein loses its native three-dimensional structure, rendering it biologically inactive. This structural disruption affects secondary, tertiary, and quaternary levels, while the primary structure (amino acid sequence) remains intact.
Causes of Denaturation:
Heat: Increases kinetic energy, disrupting hydrogen bonds and hydrophobic interactions (e.g., cooking egg whites).
pH Changes: Alters the charge distribution on amino acids, disrupting ionic bonds (e.g., stomach acid denatures ingested proteins).
Detergents: Disrupt hydrophobic interactions. Reducing Agents: Break disulfide bonds (e.g., β-mercaptoethanol).
Organic Solvents: Disrupt hydrogen bonding (e.g., alcohols).
Egg Whites: Shearing forces disrupt protein structure.
Salts: High salt concentrations disrupt ionic interactions and solubility (e.g., salting out proteins).
Examples
Example 1: Glycine ( NH2CH2COOH$ \text{NH}_2\text{CH}_2\text{COOH} $ )
Glycine is the simplest amino acid with a hydrogen atom as its R-group. It is nonpolar and small, allowing flexibility in proteins. Glycine is important in collagen and often found in tight turns of protein structures.
Example 2: Glutamic Acid ( HOOC-(CH2)2CH(NH2)COOH$ \text{HOOC-(CH}_2\text{)}_2\text{CH(NH}_2\text{)COOH} $ )
Glutamic acid is an acidic amino acid, meaning it has a carboxyl group that can donate a proton, making it negatively charged at physiological pH. It plays a key role in neurotransmission and metabolism.
Example 3: Lysine ( H2N-(CH2)4CH(NH2)COOH$ \text{H}_2\text{N-(CH}_2\text{)}_4\text{CH(NH}_2\text{)COOH} $ )
Lysine is a basic amino acid with a long hydrocarbon chain and an amino group that accepts a proton, making it positively charged. Lysine is essential in protein interactions, particularly in histones that package DNA.
Example 4: Hemoglobin
Hemoglobin is a protein with quaternary structure composed of four polypeptide subunits. It binds oxygen in the blood and transports it throughout the body. The structure of hemoglobin allows for cooperative binding, meaning oxygen binding increases its affinity for more oxygen.
Example 5: Insulin
Insulin is a peptide hormone with two polypeptide chains connected by disulfide bonds. It regulates blood sugar levels by facilitating the uptake of glucose into cells. Insulin's function depends on its precise 3D structure.
Practice Questions
Question 1
Which of the following amino acids has a basic side chain?
A) Aspartic acid
B) Serine
C) Lysine
D) Leucine
Answer: C) Lysine
Explanation: Lysine has a positively charged amino group in its side chain, making it basic. Aspartic acid is acidic, serine is polar, and leucine is nonpolar.
Question 2:
What type of bond connects amino acids in a protein's primary structure?
A) Hydrogen bond
B) Disulfide bond
C) Peptide bond
D) Ionic bond
Answer: C) Peptide bond
Explanation: Peptide bonds form between the carboxyl group of one amino acid and the amino group of another, linking them into a polypeptide chain. Hydrogen bonds and disulfide bonds stabilize higher levels of protein structure, while ionic bonds may stabilize tertiary or quaternary structures.
Question 3
What is the primary structure of a protein?
A) The sequence of amino acids
B) The local folding into alpha-helices and beta-sheets
C) The overall 3D shape of the protein
D) The arrangement of multiple polypeptide chains
Answer: A) The sequence of amino acids
Explanation: The primary structure of a protein is the linear sequence of amino acids that determines the protein's overall structure and function. Secondary, tertiary, and quaternary structures describe higher levels of folding and organization.

