The atomic nucleus is central to understanding atomic structure and plays a crucial role in nuclear physics and chemistry. On the MCAT, you’ll explore the composition of nuclei, including protons and neutrons, as well as concepts such as nuclear forces, isotopes, and decay processes. Mastering nuclear stability, radioactive decay, and the role of energy in fission and fusion reactions is essential, as these topics are integral to the chemistry and physics sections of the exam.
Learning Objectives
In studying "Atomic Nucleus" for the MCAT, you should learn to understand the structure of the nucleus, including protons and neutrons, and how they contribute to atomic mass and stability. Analyze concepts such as nuclear binding energy and mass defect, which explain the stability of nuclei. Explore different types of radioactive decay (alpha, beta, and gamma) and their roles in nuclear transformations. Evaluate how isotopes differ in nuclear composition and their significance in biological and medical applications, such as imaging and radiotherapy. Additionally, develop an understanding of half-life and radioactive decay kinetics to solve MCAT-style questions involving nuclear reactions and their applications in science and medicine.
Structure of the Nucleus: Protons and Neutrons
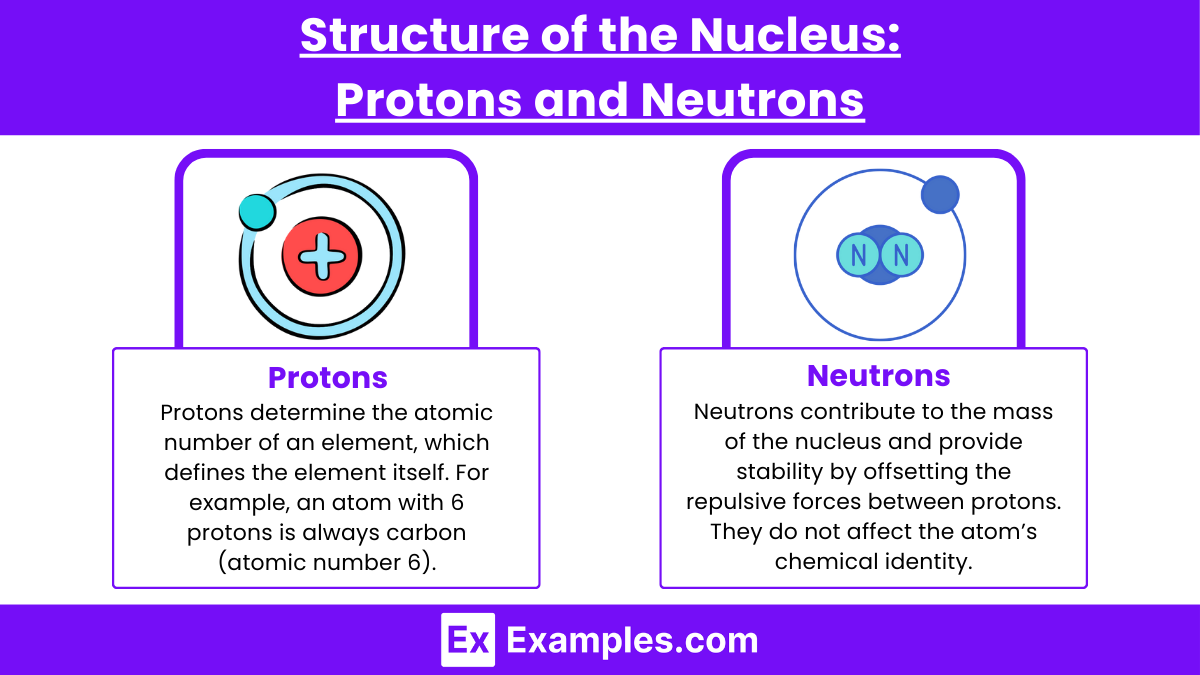
The nucleus of an atom is a small, dense region at the center, containing protons and neutrons. These particles are collectively known as nucleons and are held together by the strong nuclear force, one of the fundamental forces in nature. The structure of the nucleus determines the identity, stability, and properties of the atom.
Protons and Neutrons: The Building Blocks
Protons (p+)
Charge: +1 (positive)
Mass: 1.672×10−27 kg (about 1 atomic mass unit or amu)
Role: Protons determine the atomic number of an element, which defines the element itself. For example, an atom with 6 protons is always carbon (atomic number 6).
Interactions: Protons repel each other due to their positive charge, but the strong nuclear force binds them together inside the nucleus.
Neutrons (n0)
Charge: 0 (neutral)
Mass: 1.675×10−27 kg (slightly heavier than a proton but still about 1 amu)
Role: Neutrons contribute to the mass of the nucleus and provide stability by offsetting the repulsive forces between protons. They do not affect the atom’s chemical identity but play a crucial role in determining isotopes.
Isotopes: Atoms with the same number of protons but different numbers of neutrons. For example, carbon-12 and carbon-14 are isotopes of carbon.
Nuclear Composition and Mass
The mass number (A) of an atom is the total number of protons and neutrons in its nucleus:A = Z+N where Z is the atomic number (number of protons) and N is the number of neutrons.
Mass Defect and Binding Energy: The actual mass of the nucleus is slightly less than the sum of the masses of its individual protons and neutrons. This difference, called the mass defect, corresponds to the binding energy—the energy needed to hold the nucleons together.
Forces in the Nucleus
Strong Nuclear Force:
Acts between nucleons (protons and neutrons) to bind them together.
This force is extremely strong but acts only over very short distances (~1 femtometer).
Electrostatic Repulsion:
Protons repel each other because they have positive charges.
Neutrons help to stabilize the nucleus by adding binding force without contributing to the repulsive electrostatic force.
Nuclear Stability:
The balance between the strong nuclear force and electrostatic repulsion determines the stability of the nucleus.
Nuclei with too many or too few neutrons compared to protons can become unstable, leading to radioactive decay.
Nuclear Binding Energy and Mass Defect
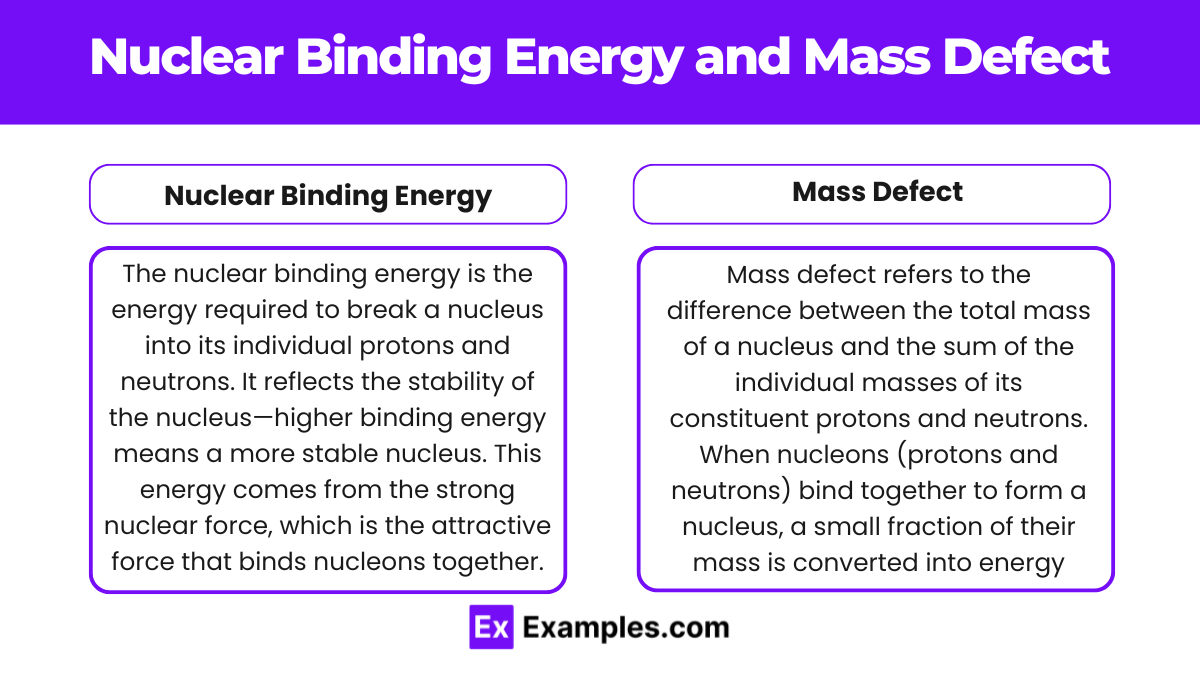
Nuclear binding energy and mass defect are two related concepts that explain the stability of atomic nuclei. They describe how energy and mass are interrelated in the context of nuclear forces, providing insights into why atomic nuclei are stable and how energy can be released in nuclear reactions.
Mass Defect
Mass defect refers to the difference between the total mass of a nucleus and the sum of the individual masses of its constituent protons and neutrons.
When nucleons (protons and neutrons) bind together to form a nucleus, a small fraction of their mass is converted into energy, following Einstein's equation: E = mc2
This lost mass is the mass defect, and it corresponds to the energy required to hold the nucleus together. The individual nucleons (when isolated) have slightly more mass than when they are bound together in the nucleus, due to the conversion of this excess mass into binding energy.
Mathematically: Δm = Zmp+(A−Z)mn−Mnucleus
where:
Δm = Mass defect
Z = Number of protons
mp = Mass of a proton
A = Mass number (total number of nucleons)
mn = Mass of a neutron
Mnucleus = Mass of the nucleus
Nuclear Binding Energy
The nuclear binding energy is the energy required to break a nucleus into its individual protons and neutrons. It reflects the stability of the nucleus—higher binding energy means a more stable nucleus. This energy comes from the strong nuclear force, which is the attractive force that binds nucleons together.
Relationship to Mass Defect: The binding energy is directly related to the mass defect. When nucleons bind together, the mass lost (Δm) is converted into binding energy (Eb).
Eb = Δm⋅c2
where:
Eb = Nuclear binding energy
Δm = Mass defect
c = Speed of light (3.00×108 m/s)
Binding Energy per Nucleon
The binding energy per nucleon is found by dividing the total binding energy by the number of nucleons in the nucleus. This value gives an idea of the stability of a nucleus:Binding energy per nucleon = Eb/A
Higher binding energy per nucleon means the nucleus is more stable.
Iron-56 and Nickel-62 have the highest binding energy per nucleon, which is why they are among the most stable elements in nature.
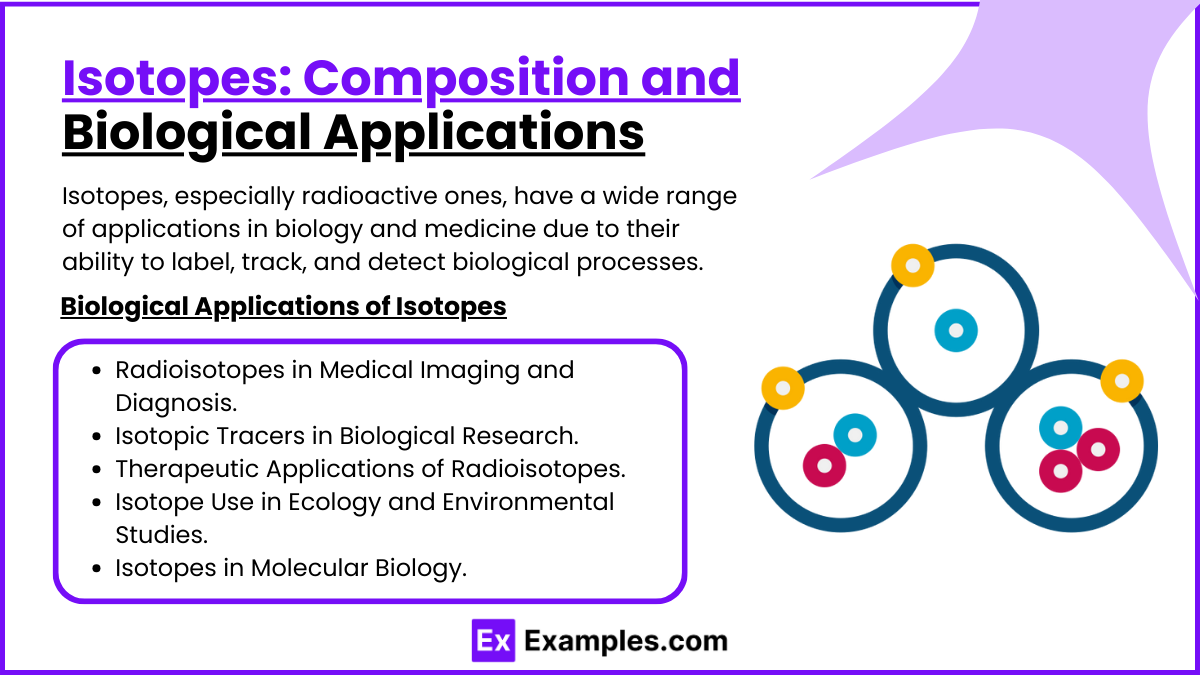
Isotopes: Composition and Biological Applications
Composition of Isotopes
Isotopes are variants of the same chemical element that have the same number of protons but different numbers of neutrons. This difference in neutron count results in isotopes having the same atomic number but different mass numbers.
Example: Carbon has several isotopes, such as:
12C: 6 protons and 6 neutrons (most common).
13C: 6 protons and 7 neutrons.
14C: 6 protons and 8 neutrons (radioactive).
Biological Applications of Isotopes
Isotopes, especially radioactive ones, have a wide range of applications in biology and medicine due to their ability to label, track, and detect biological processes.
1. Radioisotopes in Medical Imaging and Diagnosis
18F iPositron Emission Tomography (PET): Uses 18F in the form of fluorodeoxyglucose (FDG) to detect metabolic activity and diagnose cancer.
Technetium-99m (99mTc): A widely used radioisotope in nuclear medicine for imaging organs, such as the brain, heart, and kidneys.
2. Isotopic Tracers in Biological Research
Carbon-14 (14C): Used in radiocarbon dating to determine the age of organic materials.
Nitrogen-15 (15N) and Carbon-13 (13C): Used as tracers to study metabolic pathways and nutrient cycling in ecosystems.
3. Therapeutic Applications of Radioisotopes
Iodine-131 (131I): Used in the treatment of thyroid disorders, including hyperthyroidism and thyroid cancer.
Cobalt-60 (60Co): Employed in radiotherapy to treat cancer by emitting gamma rays that target tumor cells.
4. Isotope Use in Ecology and Environmental Studies
Stable Isotope Analysis: Stable isotopes of elements such as hydrogen (2H) and oxygen (18O) are used to track animal migration, water sources, and climate change effects.
Isotope Ratios: Analysis of isotope ratios (e.g., 13C/12C) in plant tissues provides insights into photosynthetic pathways (C3 vs. C4 plants).
5. Isotopes in Molecular Biology
Phosphorus-32 (32P): Used to label DNA or RNA molecules in molecular biology experiments to study replication, transcription, and translation processes.
Examples
Example 1: Structure of Helium Nucleus
The helium nucleus, also known as an alpha particle, consists of two protons and two neutrons. This simple structure exemplifies the fundamental composition of atomic nuclei and highlights the strong nuclear force that holds these particles together. Helium nuclei play a crucial role in nuclear fusion processes, such as those occurring in stars, where they are formed from hydrogen nuclei.
Example 2: Isotopes of Carbon
The carbon atom has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. These isotopes differ in the number of neutrons present in their atomic nuclei. For example, carbon-12 has six protons and six neutrons, while carbon-14 has six protons and eight neutrons. The study of these isotopes is essential in fields such as archaeology, where carbon-14 dating helps determine the age of ancient organic materials.
Example 3: Nuclear Fission in Uranium
In nuclear power plants, the fission of uranium-235 nuclei is a critical process for generating energy. When a uranium nucleus absorbs a neutron, it becomes unstable and splits into smaller nuclei, releasing a significant amount of energy and additional neutrons. This chain reaction is harnessed to produce electricity, demonstrating the practical applications of understanding the atomic nucleus in energy production.
Example 4: Nuclear Medicine
The atomic nucleus is fundamental in the field of nuclear medicine, where radioactive isotopes are used for diagnostic imaging and treatment. For instance, technetium-99m, a radioactive isotope, is widely used in medical imaging to visualize organs and detect abnormalities. The behavior of the atomic nucleus in this context is crucial for developing effective imaging techniques and therapies.
Example 5: Alpha Decay
Alpha decay is a type of radioactive decay that occurs when an atomic nucleus emits an alpha particle, which consists of two protons and two neutrons. This process reduces the atomic number of the original nucleus, transforming it into a different element. For example, when uranium-238 undergoes alpha decay, it transforms into thorium-234. Understanding alpha decay is important in studying the stability of atomic nuclei and the behavior of radioactive materials in nature.
Practice Questions
Question 1
What are the two primary particles found in the atomic nucleus?
A) Protons and electrons
B) Neutrons and electrons
C) Protons and neutrons
D) Electrons and positrons
Correct Answer: C) Protons and neutrons.
Explanation: The atomic nucleus is composed of protons and neutrons, collectively known as nucleons. Protons carry a positive charge, while neutrons are neutral. Electrons, which are negatively charged, orbit the nucleus but are not part of it. Understanding the composition of the nucleus is fundamental to grasping concepts such as atomic structure and isotopes.
Question 2
What is the significance of the number of protons in an atomic nucleus?
A) It determines the mass of the atom.
B) It defines the chemical properties and identity of the element.
C) It indicates the number of neutrons present.
D) It has no significance in atomic theory.
Correct Answer: B) It defines the chemical properties and identity of the element.
Explanation: The number of protons in the nucleus, known as the atomic number, uniquely identifies an element and determines its chemical properties. For instance, hydrogen has one proton, while carbon has six. The atomic number also dictates the element's position in the periodic table. Options A and C are incorrect because the mass is affected by both protons and neutrons, and the number of neutrons can vary independently of the number of protons in isotopes.
Question 3
Which of the following describes a stable atomic nucleus?
A) A nucleus that has a high ratio of protons to neutrons.
B) A nucleus that has equal numbers of protons and neutrons.
C) A nucleus that is not undergoing radioactive decay.
D) A nucleus that contains only neutrons.
Correct Answer: C) A nucleus that is not undergoing radioactive decay.
Explanation: A stable atomic nucleus is characterized by its ability to maintain its composition without undergoing radioactive decay. Stability often depends on a balanced ratio of protons to neutrons, but it is not solely defined by having equal numbers of these particles. While many stable nuclei have a specific ratio of protons to neutrons, the key factor is their resistance to decay. Options A and D are misleading because a high proton-to-neutron ratio can lead to instability, and a nucleus with only neutrons would not be stable as it lacks protons.