Carboxylic acid Derivatives

- Notes
Carboxylic acid derivatives are essential compounds in organic chemistry and biochemistry, playing a vital role in both synthetic and biological processes. These derivatives include acid chlorides, anhydrides, esters, amides, and thioesters, all containing a carbonyl group bonded to different substituents. Their reactivity varies based on the leaving group, influencing their use in reactions like hydrolysis, esterification, and amidation.
Learning Objectives
In studying “Carboxylic Acid Derivatives” for the MCAT, you should learn to identify and understand the structure, reactivity, and synthesis of acid chlorides, anhydrides, esters, amides, and thioesters. Analyze how these derivatives interconvert through nucleophilic acyl substitution reactions and evaluate their hydrolysis under acidic and basic conditions. Explore their biological significance, including peptide bonds in proteins, ester linkages in triglycerides, and thioesters in acetyl-CoA. Additionally, understand their role in pharmaceuticals, such as aspirin synthesis, and apply this knowledge to interpret reaction mechanisms and predict products in MCAT-style passages and questions.
Carboxylic acid derivatives are organic compounds that contain a carbonyl group (C = O) bonded to an electronegative atom or group. Understanding their properties, synthesis, reactivity, and interconversion is essential for mastering organic chemistry concepts relevant to the MCAT.
Types of Carboxylic Acid Derivatives
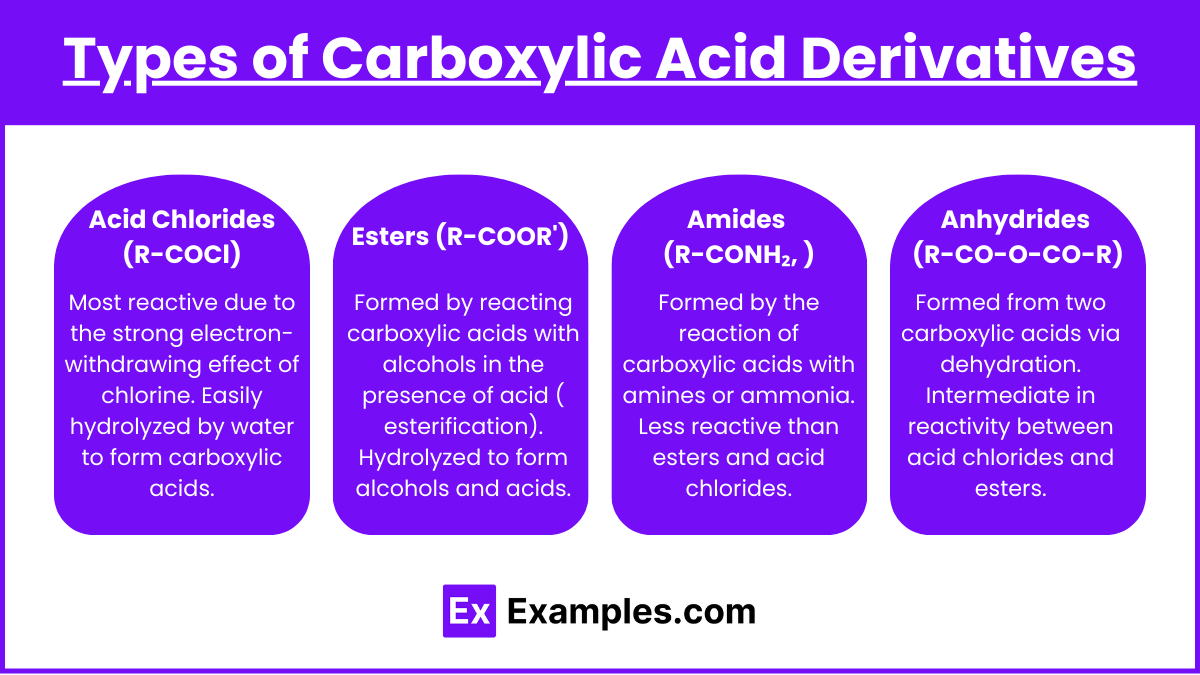
- Acid Chlorides (R-COCl)
- Most reactive due to the strong electron-withdrawing effect of chlorine.
- Easily hydrolyzed by water to form carboxylic acids.
- Esters (R-COOR’)
- Formed by reacting carboxylic acids with alcohols in the presence of acid (Fischer esterification).
- Hydrolyzed to form alcohols and acids.
- Amides (R-CONH₂, R-CONHR’, R-CONR₂’)
- Formed by the reaction of carboxylic acids with amines or ammonia.
- Less reactive than esters and acid chlorides.
- Anhydrides (R-CO-O-CO-R)
- Formed from two carboxylic acids via dehydration.
- Intermediate in reactivity between acid chlorides and esters.
Reactivity and Interconversion of Carboxylic Acid Derivatives

The reactivity of these derivatives depends on the leaving group attached to the carbonyl. The general trend of reactivity is:
Acid Chloride > Anhydride > Ester > Amide
- Hydrolysis: All derivatives can convert back into carboxylic acids using water, though the conditions vary:
- Acid chlorides hydrolyze rapidly in water.
- Esters require acidic or basic conditions for hydrolysis.
- Amides need strong acidic or basic hydrolysis.
- Transesterification: Esters can be converted into other esters by reacting with alcohols under acidic or basic conditions.
- Reduction:
- Esters and amides can be reduced to alcohols using strong reducing agents like LiAlH₄ (Lithium Aluminum Hydride).
- Anhydrides and acid chlorides also reduce to alcohols or aldehydes depending on conditions.
Properties and Mechanisms
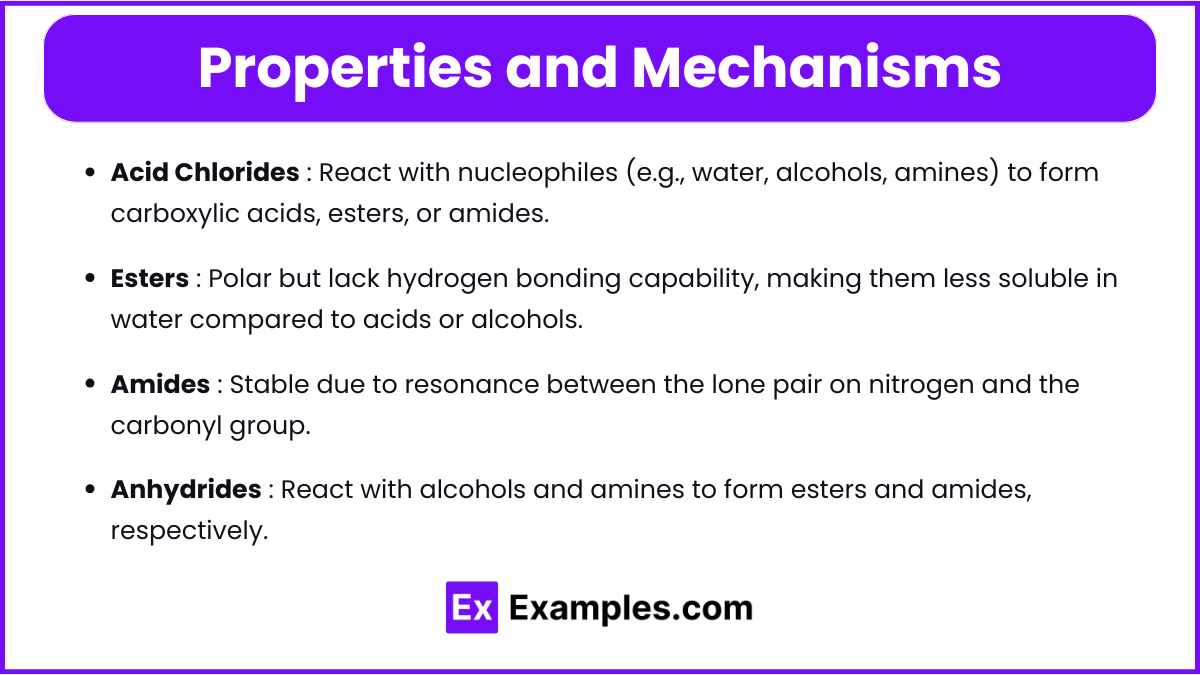
- Acid Chlorides
- React with nucleophiles (e.g., water, alcohols, amines) to form carboxylic acids, esters, or amides.
- Used in synthesis due to their high reactivity.
- Esters
- Polar but lack hydrogen bonding capability, making them less soluble in water compared to acids or alcohols.
- Undergo nucleophilic acyl substitution reactions.
- Amides
- Stable due to resonance between the lone pair on nitrogen and the carbonyl group.
- Exhibit high boiling points due to hydrogen bonding.
- Anhydrides
- React with alcohols and amines to form esters and amides, respectively.
- Easily hydrolyzed in the presence of water.
Biological Importance
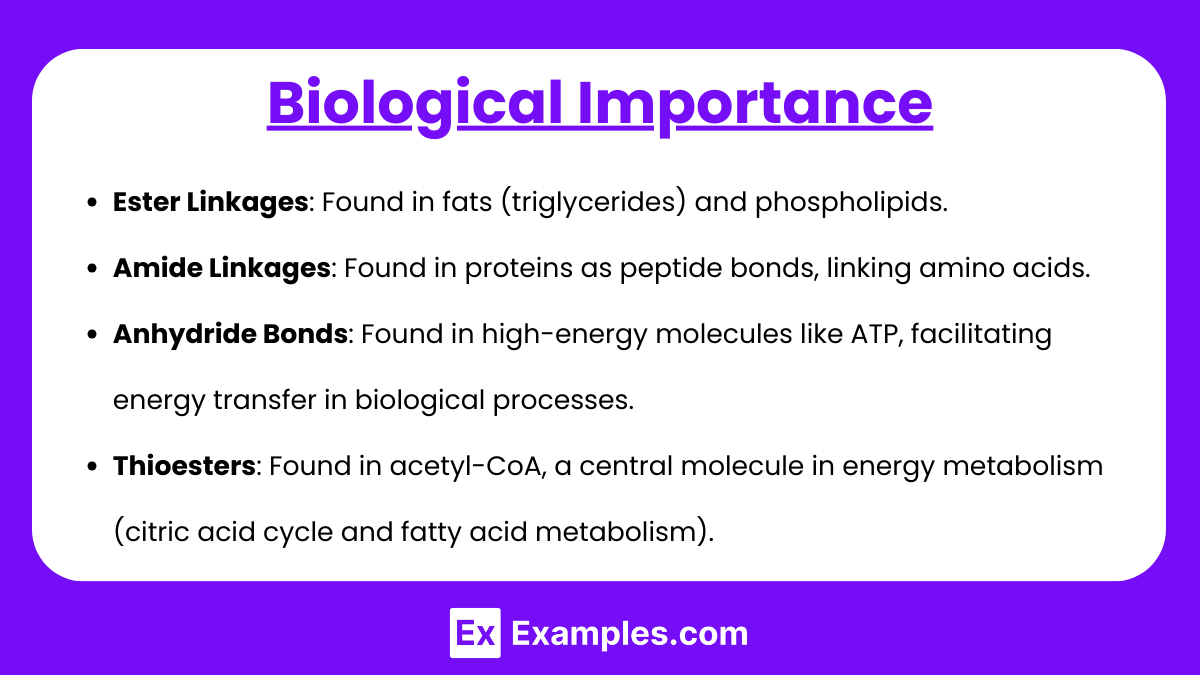
- Ester Linkages: Found in fats (triglycerides) and phospholipids.
- Amide Linkages: Found in proteins as peptide bonds, linking amino acids.
- Anhydride Bonds: Found in high-energy molecules like ATP, facilitating energy transfer in biological processes.
- Thioesters: Found in acetyl-CoA, a central molecule in energy metabolism (citric acid cycle and fatty acid metabolism).
- Lactones and Lactams (Cyclic Esters and Amides): Lactones are found in natural products like vitamin C (ascorbic acid) and plant-derived secondary metabolites.
Examples
Examples of Carboxylic Acid Derivatives
Example 1: Triglycerides (Fats and Oils)
Triglycerides are esters formed from three fatty acids bonded to a glycerol molecule. They serve as the primary storage form of energy in animals and plants. Upon hydrolysis, triglycerides release free fatty acids and glycerol, which can enter metabolic pathways like β-oxidation for energy production.
Example 2: Phospholipids
Phospholipids are derivatives of carboxylic acids that contain two fatty acids esterified to glycerol, along with a phosphate group on the third position. These molecules are crucial components of cell membranes, contributing to membrane fluidity and facilitating cell signaling through lipid-based signaling pathways.
Example 3 : Peptide Bonds in Proteins
Amide linkages, known as peptide bonds, are formed between the carboxyl group of one amino acid and the amine group of another during protein synthesis. These bonds hold amino acids together in polypeptide chains, giving rise to proteins that perform structural, enzymatic, and regulatory roles within cells.
Example 4 : Acetyl-CoA (Thioester)
Acetyl-CoA contains a thioester bond between acetic acid and coenzyme A. It plays a central role in metabolism, acting as a key intermediate in the citric acid cycle (Krebs cycle) and fatty acid synthesis. Acetyl-CoA also serves as an acetyl donor in various biochemical reactions, including histone acetylation.
Example 5 : Aspirin (Acetylsalicylic Acid)
Aspirin is an example of a synthetic derivative of carboxylic acid. It is prepared by acetylating salicylic acid with acetic anhydride, resulting in an ester linkage. As an anti-inflammatory drug, aspirin irreversibly inhibits cyclooxygenase (COX) enzymes, reducing the production of prostaglandins involved in pain and inflammation.
Practice Questions
Question 1
Which of the following carboxylic acid derivatives is the most reactive toward nucleophilic attack?
A) Amides
B) Esters
C) Acid Anhydrides
D) Acid Chlorides
Correct Answer: D) Acid Chlorides
Explanation: The reactivity of carboxylic acid derivatives depends on the leaving group’s stability and the electrophilicity of the carbonyl carbon. Acid chlorides are the most reactive due to the strong electron-withdrawing effect of chlorine, which makes the carbonyl carbon more electrophilic. Additionally, chloride ions are good leaving groups, which enhances the compound’s reactivity. In comparison, amides are the least reactive because the nitrogen group donates electron density to the carbonyl carbon, stabilizing the structure through resonance.
Question 2
Which reaction describes the conversion of an ester to a carboxylic acid under acidic or basic conditions?
A) Transesterification
B) Esterification
C) Hydrolysis
D) Amidation
Correct Answer: C) Hydrolysis
Explanation:
Hydrolysis is the process of breaking down an ester into a carboxylic acid and an alcohol using water. Under acidic conditions, the ester is protonated, making it more susceptible to nucleophilic attack by water. Under basic conditions, hydroxide ions attack the carbonyl carbon, leading to the formation of a carboxylate ion, which is then protonated to give a carboxylic acid. Transesterification refers to the conversion of one ester to another, and esterification involves the formation of an ester from a carboxylic acid and an alcohol. Amidation refers to the formation of an amide.
Question 3
Which of the following is a biological example of a thioester?
A) Phospholipids
B) Peptide Bonds
C) Acetyl-CoA
D) Triglycerides
Correct Answer: C) Acetyl-CoA
Explanation: Acetyl-CoA is a molecule that contains a thioester bond between an acyl group (acetate) and coenzyme A. Thioesters are highly reactive due to the sulfur atom, which makes them critical intermediates in metabolic processes such as the citric acid cycle and fatty acid synthesis. In contrast, peptide bonds (amide linkages) form the primary structure of proteins, triglycerides contain ester bonds, and phospholipids have both ester and phosphate linkages but no thioester bonds.
Carboxylic acid derivatives are essential compounds in organic chemistry and biochemistry, playing a vital role in both synthetic and biological processes. These derivatives include acid chlorides, anhydrides, esters, amides, and thioesters, all containing a carbonyl group bonded to different substituents. Their reactivity varies based on the leaving group, influencing their use in reactions like hydrolysis, esterification, and amidation.
Learning Objectives
In studying "Carboxylic Acid Derivatives" for the MCAT, you should learn to identify and understand the structure, reactivity, and synthesis of acid chlorides, anhydrides, esters, amides, and thioesters. Analyze how these derivatives interconvert through nucleophilic acyl substitution reactions and evaluate their hydrolysis under acidic and basic conditions. Explore their biological significance, including peptide bonds in proteins, ester linkages in triglycerides, and thioesters in acetyl-CoA. Additionally, understand their role in pharmaceuticals, such as aspirin synthesis, and apply this knowledge to interpret reaction mechanisms and predict products in MCAT-style passages and questions.
Carboxylic acid derivatives are organic compounds that contain a carbonyl group (C = O) bonded to an electronegative atom or group. Understanding their properties, synthesis, reactivity, and interconversion is essential for mastering organic chemistry concepts relevant to the MCAT.
Types of Carboxylic Acid Derivatives

Acid Chlorides (R-COCl)
Most reactive due to the strong electron-withdrawing effect of chlorine.
Easily hydrolyzed by water to form carboxylic acids.
Esters (R-COOR')
Formed by reacting carboxylic acids with alcohols in the presence of acid (Fischer esterification).
Hydrolyzed to form alcohols and acids.
Amides (R-CONH₂, R-CONHR', R-CONR₂')
Formed by the reaction of carboxylic acids with amines or ammonia.
Less reactive than esters and acid chlorides.
Anhydrides (R-CO-O-CO-R)
Formed from two carboxylic acids via dehydration.
Intermediate in reactivity between acid chlorides and esters.
Reactivity and Interconversion of Carboxylic Acid Derivatives

The reactivity of these derivatives depends on the leaving group attached to the carbonyl. The general trend of reactivity is:
Acid Chloride > Anhydride > Ester > Amide
Hydrolysis: All derivatives can convert back into carboxylic acids using water, though the conditions vary:
Acid chlorides hydrolyze rapidly in water.
Esters require acidic or basic conditions for hydrolysis.
Amides need strong acidic or basic hydrolysis.
Transesterification: Esters can be converted into other esters by reacting with alcohols under acidic or basic conditions.
Reduction:
Esters and amides can be reduced to alcohols using strong reducing agents like LiAlH₄ (Lithium Aluminum Hydride).
Anhydrides and acid chlorides also reduce to alcohols or aldehydes depending on conditions.
Properties and Mechanisms

Acid Chlorides
React with nucleophiles (e.g., water, alcohols, amines) to form carboxylic acids, esters, or amides.
Used in synthesis due to their high reactivity.
Esters
Polar but lack hydrogen bonding capability, making them less soluble in water compared to acids or alcohols.
Undergo nucleophilic acyl substitution reactions.
Amides
Stable due to resonance between the lone pair on nitrogen and the carbonyl group.
Exhibit high boiling points due to hydrogen bonding.
Anhydrides
React with alcohols and amines to form esters and amides, respectively.
Easily hydrolyzed in the presence of water.
Biological Importance

Ester Linkages: Found in fats (triglycerides) and phospholipids.
Amide Linkages: Found in proteins as peptide bonds, linking amino acids.
Anhydride Bonds: Found in high-energy molecules like ATP, facilitating energy transfer in biological processes.
Thioesters: Found in acetyl-CoA, a central molecule in energy metabolism (citric acid cycle and fatty acid metabolism).
Lactones and Lactams (Cyclic Esters and Amides): Lactones are found in natural products like vitamin C (ascorbic acid) and plant-derived secondary metabolites.
Examples
Examples of Carboxylic Acid Derivatives
Example 1: Triglycerides (Fats and Oils)
Triglycerides are esters formed from three fatty acids bonded to a glycerol molecule. They serve as the primary storage form of energy in animals and plants. Upon hydrolysis, triglycerides release free fatty acids and glycerol, which can enter metabolic pathways like β-oxidation for energy production.
Example 2: Phospholipids
Phospholipids are derivatives of carboxylic acids that contain two fatty acids esterified to glycerol, along with a phosphate group on the third position. These molecules are crucial components of cell membranes, contributing to membrane fluidity and facilitating cell signaling through lipid-based signaling pathways.
Example 3 : Peptide Bonds in Proteins
Amide linkages, known as peptide bonds, are formed between the carboxyl group of one amino acid and the amine group of another during protein synthesis. These bonds hold amino acids together in polypeptide chains, giving rise to proteins that perform structural, enzymatic, and regulatory roles within cells.
Example 4 : Acetyl-CoA (Thioester)
Acetyl-CoA contains a thioester bond between acetic acid and coenzyme A. It plays a central role in metabolism, acting as a key intermediate in the citric acid cycle (Krebs cycle) and fatty acid synthesis. Acetyl-CoA also serves as an acetyl donor in various biochemical reactions, including histone acetylation.
Example 5 : Aspirin (Acetylsalicylic Acid)
Aspirin is an example of a synthetic derivative of carboxylic acid. It is prepared by acetylating salicylic acid with acetic anhydride, resulting in an ester linkage. As an anti-inflammatory drug, aspirin irreversibly inhibits cyclooxygenase (COX) enzymes, reducing the production of prostaglandins involved in pain and inflammation.
Practice Questions
Question 1
Which of the following carboxylic acid derivatives is the most reactive toward nucleophilic attack?
A) Amides
B) Esters
C) Acid Anhydrides
D) Acid Chlorides
Correct Answer: D) Acid Chlorides
Explanation: The reactivity of carboxylic acid derivatives depends on the leaving group’s stability and the electrophilicity of the carbonyl carbon. Acid chlorides are the most reactive due to the strong electron-withdrawing effect of chlorine, which makes the carbonyl carbon more electrophilic. Additionally, chloride ions are good leaving groups, which enhances the compound’s reactivity. In comparison, amides are the least reactive because the nitrogen group donates electron density to the carbonyl carbon, stabilizing the structure through resonance.
Question 2
Which reaction describes the conversion of an ester to a carboxylic acid under acidic or basic conditions?
A) Transesterification
B) Esterification
C) Hydrolysis
D) Amidation
Correct Answer: C) Hydrolysis
Explanation:
Hydrolysis is the process of breaking down an ester into a carboxylic acid and an alcohol using water. Under acidic conditions, the ester is protonated, making it more susceptible to nucleophilic attack by water. Under basic conditions, hydroxide ions attack the carbonyl carbon, leading to the formation of a carboxylate ion, which is then protonated to give a carboxylic acid. Transesterification refers to the conversion of one ester to another, and esterification involves the formation of an ester from a carboxylic acid and an alcohol. Amidation refers to the formation of an amide.
Question 3
Which of the following is a biological example of a thioester?
A) Phospholipids
B) Peptide Bonds
C) Acetyl-CoA
D) Triglycerides
Correct Answer: C) Acetyl-CoA
Explanation: Acetyl-CoA is a molecule that contains a thioester bond between an acyl group (acetate) and coenzyme A. Thioesters are highly reactive due to the sulfur atom, which makes them critical intermediates in metabolic processes such as the citric acid cycle and fatty acid synthesis. In contrast, peptide bonds (amide linkages) form the primary structure of proteins, triglycerides contain ester bonds, and phospholipids have both ester and phosphate linkages but no thioester bonds.

