Carboxylic Acids

- Notes
Carboxylic acids are a significant class of organic compounds characterized by the presence of a carboxyl functional group (-COOH). These acids are commonly found in nature and play a vital role in various biological and chemical processes. For the MCAT, understanding the structure, properties, and reactivity of carboxylic acids is essential, as they form the basis of many reactions, including oxidation, reduction, and the formation of derivatives like esters and amides. Familiarity with carboxylic acids aids in grasping broader concepts in biochemistry and organic chemistry.
Learning Objectives
When studying “Carboxylic Acids” for the MCAT, focus on their structure, particularly the carboxyl group (-COOH), and how it influences properties like acidity, polarity, and boiling points. Study how substituents and resonance affect their acidity. Understand key reactions, such as nucleophilic acyl substitution and esterification, and the formation of derivatives like esters, amides, and anhydrides. Mastering these concepts will help you handle related questions effectively in the MCAT’s organic chemistry and biochemistry sections.
1. Introduction to Carboxylic Acids
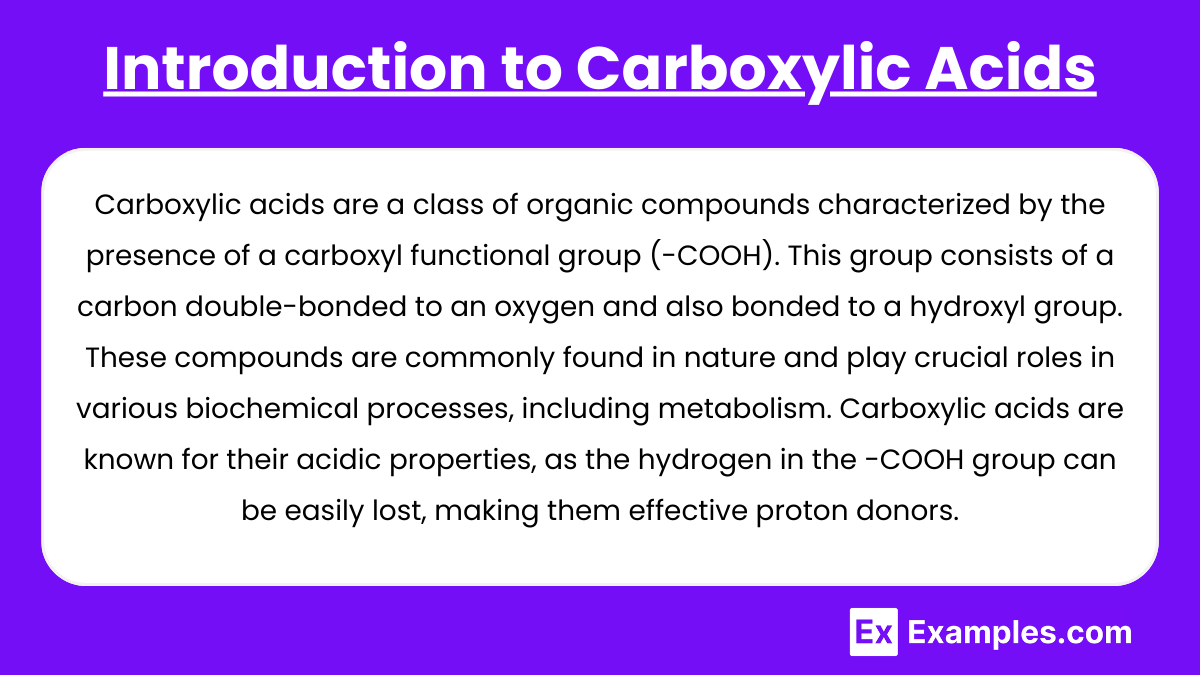
Carboxylic acids are a class of organic compounds characterized by the presence of a carboxyl functional group (-COOH). This group consists of a carbon double-bonded to an oxygen and also bonded to a hydroxyl group. These compounds are commonly found in nature and play crucial roles in various biochemical processes, including metabolism. Carboxylic acids are known for their acidic properties, as the hydrogen in the -COOH group can be easily lost, making them effective proton donors. They are widely used in the synthesis of pharmaceuticals, polymers, and food preservatives, reflecting their versatility in both biological and industrial applications.
2. Structure of Carboxylic Acids
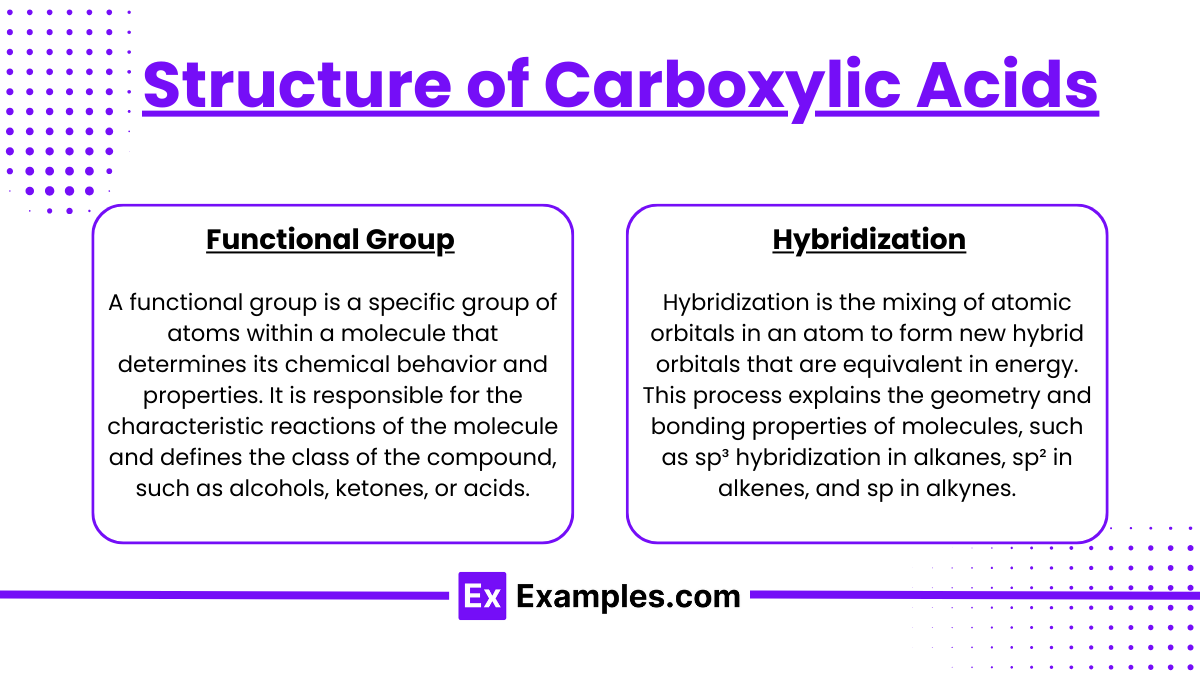
- Functional Group: The carboxyl group consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group.
- Hybridization: The carbon in the carboxyl group is sp² hybridized, leading to a trigonal planar structure.
- Examples: Common carboxylic acids include acetic acid (CH₃COOH), citric acid (C₆H₈O₇), and formic acid (HCOOH).
3. Key Reactions Involving Carboxylic Acids
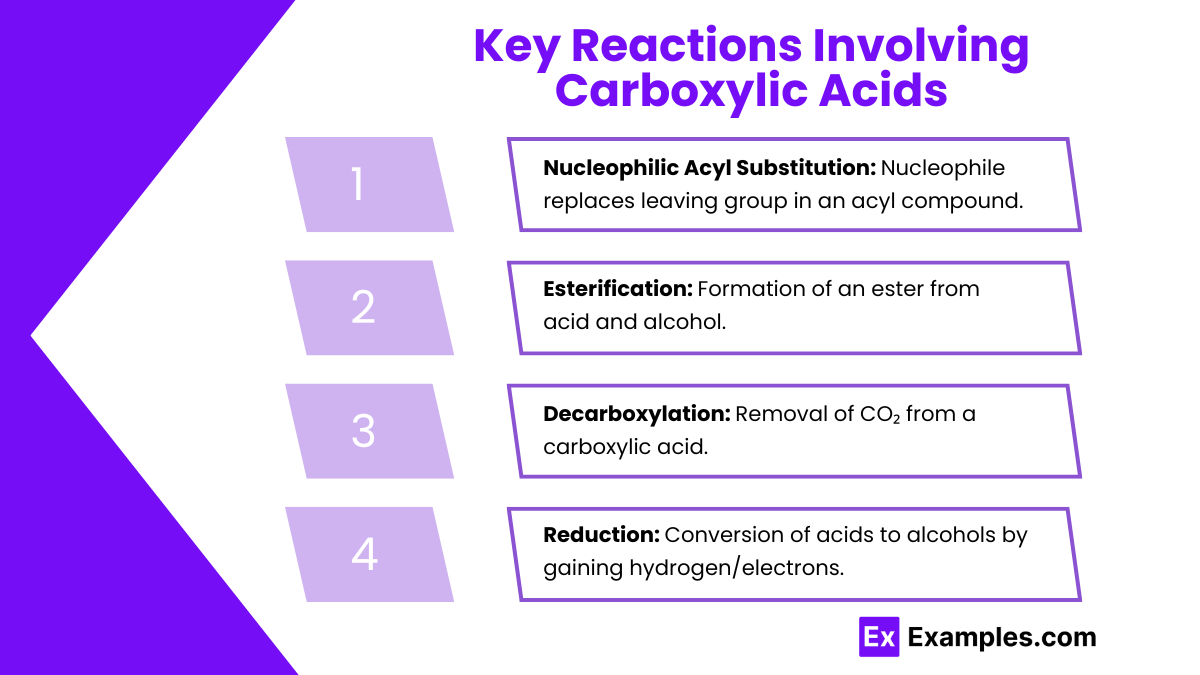
- Nucleophilic Acyl Substitution:
- Mechanism: A nucleophile attacks the carbonyl carbon, leading to the formation of a tetrahedral intermediate, followed by the elimination of a leaving group.
- Products: This reaction produces derivatives like esters and amides.
- Esterification:
- Reaction with Alcohols: Carboxylic acids react with alcohols in the presence of an acid catalyst to form esters.
- Example: Acetic acid + Ethanol → Ethyl acetate + Water.
- Decarboxylation:
- Loss of CO₂: Decarboxylation involves the removal of a carbon dioxide molecule, often facilitated by heat.
- Importance: This reaction is crucial in various metabolic pathways, including the citric acid cycle.
- Reduction:
- Carboxylic acids can be reduced to primary alcohols using reducing agents like lithium aluminum hydride (LiAlH₄).
Examples
Example 1. Synthesis of Esters
Carboxylic acids are commonly used in the synthesis of esters through a reaction known as esterification. In this process, a carboxylic acid reacts with an alcohol in the presence of an acid catalyst, usually sulfuric acid. This reaction produces an ester and water. For example, the reaction of acetic acid with ethanol yields ethyl acetate, a solvent widely used in laboratories and the production of nail polish remover. This reaction is significant in organic chemistry, illustrating the transformation of functional groups.
Example 2. Biological Metabolism
Carboxylic acids play a crucial role in biological metabolism, particularly in the citric acid cycle (Krebs cycle). In this cycle, citric acid, a tricarboxylic acid, is formed from acetyl-CoA and oxaloacetate. The subsequent reactions generate energy through the oxidation of substrates. This pathway is vital for cellular respiration, as it contributes to the production of ATP, the energy currency of the cell. Understanding this process is essential for grasping metabolic functions in biochemistry.
Example 3. Food Industry Applications
Carboxylic acids are widely used in the food industry as preservatives and flavoring agents. For instance, acetic acid is a key component of vinegar, which not only adds flavor but also acts as a preservative due to its antimicrobial properties. Additionally, citric acid is frequently used in food and beverages to provide a sour taste and as a natural preservative. These acids help maintain food safety and enhance sensory qualities, making them invaluable in food science.
Example 4. Production of Soaps and Detergents
Carboxylic acids are fundamental in the production of soaps and detergents. The saponification process involves the reaction of a fat or oil (triglyceride) with a strong base, such as sodium hydroxide (NaOH), resulting in the formation of glycerol and fatty acid salts, which are the active ingredients in soaps. For example, the saponification of stearic acid produces sodium stearate, a common ingredient in many cleaning products. This application highlights the importance of carboxylic acids in everyday hygiene products.
Example 5. Pharmaceuticals and Drug Synthesis
Carboxylic acids are crucial in the synthesis of various pharmaceuticals. Many drugs contain carboxylic acid functional groups, which can influence their biological activity and solubility. For example, ibuprofen, a widely used nonsteroidal anti-inflammatory drug (NSAID), contains a carboxylic acid group that contributes to its effectiveness in reducing pain and inflammation. Understanding how to manipulate carboxylic acids in drug design is essential for pharmaceutical chemistry and medicinal applications.
Practice Questions
Question 1
Which of the following carboxylic acids is the strongest?
A) Acetic acid (CH₃COOH)
B) Formic acid (HCOOH)
C) Benzoic acid (C₆H₅COOH)
D) Propanoic acid (C₂H₅COOH)
Answer: B) Formic acid (HCOOH)
Explanation: Formic acid is stronger than acetic acid and propanoic acid due to the absence of an electron-donating alkyl group. The acidity of carboxylic acids is influenced by the stability of their conjugate bases. Formic acid’s conjugate base (formate ion) is more stable than that of acetic acid (acetate ion) because the negative charge on the formate ion is less destabilized by resonance compared to the acetate ion, which has an alkyl group that can push electron density toward the carboxylate. Benzoic acid, while it has a resonance-stabilized conjugate base, is influenced by the electron-donating effects of the phenyl group, making it less acidic than formic acid.
Question 2
What is the main product formed when butanoic acid reacts with an excess of ethanol in the presence of sulfuric acid?
A) Butanoate
B) Ethyl butanoate
C) Butanol
D) Butyl acetate
Answer: B) Ethyl butanoate
Explanation: The reaction between butanoic acid and ethanol in the presence of sulfuric acid is an esterification reaction, specifically Fischer esterification. The sulfuric acid acts as a catalyst to promote the reaction between the carboxylic acid (butanoic acid) and the alcohol (ethanol). The resulting product, ethyl butanoate, is an ester formed from the hydroxyl group of the acid and the hydrogen from the alcohol. The excess ethanol ensures a higher yield of the ester product by driving the reaction towards the formation of ethyl butanoate and releasing water as a byproduct.
Question 3
Which statement about carboxylic acids is FALSE?
A) Carboxylic acids can form hydrogen bonds with water.
B) The boiling point of carboxylic acids is lower than that of alcohols.
C) Carboxylic acids can undergo decarboxylation.
D) Carboxylic acids are generally more acidic than alcohols.
Answer: B) The boiling point of carboxylic acids is lower than that of alcohols.
Explanation: This statement is false because carboxylic acids typically have higher boiling points than alcohols due to their ability to form strong hydrogen bonds, both with each other and with water. The presence of the carboxyl group (-COOH) allows for more extensive hydrogen bonding compared to the hydroxyl group (-OH) in alcohols. This leads to increased boiling points for carboxylic acids relative to alcohols of similar molecular weight. The other statements are true: carboxylic acids do form hydrogen bonds with water, they can undergo decarboxylation (loss of CO₂), and they are more acidic than alcohols due to the stability of their conjugate bases.
Carboxylic acids are a significant class of organic compounds characterized by the presence of a carboxyl functional group (-COOH). These acids are commonly found in nature and play a vital role in various biological and chemical processes. For the MCAT, understanding the structure, properties, and reactivity of carboxylic acids is essential, as they form the basis of many reactions, including oxidation, reduction, and the formation of derivatives like esters and amides. Familiarity with carboxylic acids aids in grasping broader concepts in biochemistry and organic chemistry.
Learning Objectives
When studying "Carboxylic Acids" for the MCAT, focus on their structure, particularly the carboxyl group (-COOH), and how it influences properties like acidity, polarity, and boiling points. Study how substituents and resonance affect their acidity. Understand key reactions, such as nucleophilic acyl substitution and esterification, and the formation of derivatives like esters, amides, and anhydrides. Mastering these concepts will help you handle related questions effectively in the MCAT’s organic chemistry and biochemistry sections.
1. Introduction to Carboxylic Acids

Carboxylic acids are a class of organic compounds characterized by the presence of a carboxyl functional group (-COOH). This group consists of a carbon double-bonded to an oxygen and also bonded to a hydroxyl group. These compounds are commonly found in nature and play crucial roles in various biochemical processes, including metabolism. Carboxylic acids are known for their acidic properties, as the hydrogen in the -COOH group can be easily lost, making them effective proton donors. They are widely used in the synthesis of pharmaceuticals, polymers, and food preservatives, reflecting their versatility in both biological and industrial applications.
2. Structure of Carboxylic Acids

Functional Group: The carboxyl group consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group.
Hybridization: The carbon in the carboxyl group is sp² hybridized, leading to a trigonal planar structure.
Examples: Common carboxylic acids include acetic acid (CH₃COOH), citric acid (C₆H₈O₇), and formic acid (HCOOH).
3. Key Reactions Involving Carboxylic Acids

Nucleophilic Acyl Substitution:
Mechanism: A nucleophile attacks the carbonyl carbon, leading to the formation of a tetrahedral intermediate, followed by the elimination of a leaving group.
Products: This reaction produces derivatives like esters and amides.
Esterification:
Reaction with Alcohols: Carboxylic acids react with alcohols in the presence of an acid catalyst to form esters.
Example: Acetic acid + Ethanol → Ethyl acetate + Water.
Decarboxylation:
Loss of CO₂: Decarboxylation involves the removal of a carbon dioxide molecule, often facilitated by heat.
Importance: This reaction is crucial in various metabolic pathways, including the citric acid cycle.
Reduction:
Carboxylic acids can be reduced to primary alcohols using reducing agents like lithium aluminum hydride (LiAlH₄).
Examples
Example 1. Synthesis of Esters
Carboxylic acids are commonly used in the synthesis of esters through a reaction known as esterification. In this process, a carboxylic acid reacts with an alcohol in the presence of an acid catalyst, usually sulfuric acid. This reaction produces an ester and water. For example, the reaction of acetic acid with ethanol yields ethyl acetate, a solvent widely used in laboratories and the production of nail polish remover. This reaction is significant in organic chemistry, illustrating the transformation of functional groups.
Example 2. Biological Metabolism
Carboxylic acids play a crucial role in biological metabolism, particularly in the citric acid cycle (Krebs cycle). In this cycle, citric acid, a tricarboxylic acid, is formed from acetyl-CoA and oxaloacetate. The subsequent reactions generate energy through the oxidation of substrates. This pathway is vital for cellular respiration, as it contributes to the production of ATP, the energy currency of the cell. Understanding this process is essential for grasping metabolic functions in biochemistry.
Example 3. Food Industry Applications
Carboxylic acids are widely used in the food industry as preservatives and flavoring agents. For instance, acetic acid is a key component of vinegar, which not only adds flavor but also acts as a preservative due to its antimicrobial properties. Additionally, citric acid is frequently used in food and beverages to provide a sour taste and as a natural preservative. These acids help maintain food safety and enhance sensory qualities, making them invaluable in food science.
Example 4. Production of Soaps and Detergents
Carboxylic acids are fundamental in the production of soaps and detergents. The saponification process involves the reaction of a fat or oil (triglyceride) with a strong base, such as sodium hydroxide (NaOH), resulting in the formation of glycerol and fatty acid salts, which are the active ingredients in soaps. For example, the saponification of stearic acid produces sodium stearate, a common ingredient in many cleaning products. This application highlights the importance of carboxylic acids in everyday hygiene products.
Example 5. Pharmaceuticals and Drug Synthesis
Carboxylic acids are crucial in the synthesis of various pharmaceuticals. Many drugs contain carboxylic acid functional groups, which can influence their biological activity and solubility. For example, ibuprofen, a widely used nonsteroidal anti-inflammatory drug (NSAID), contains a carboxylic acid group that contributes to its effectiveness in reducing pain and inflammation. Understanding how to manipulate carboxylic acids in drug design is essential for pharmaceutical chemistry and medicinal applications.
Practice Questions
Question 1
Which of the following carboxylic acids is the strongest?
A) Acetic acid (CH₃COOH)
B) Formic acid (HCOOH)
C) Benzoic acid (C₆H₅COOH)
D) Propanoic acid (C₂H₅COOH)
Answer: B) Formic acid (HCOOH)
Explanation: Formic acid is stronger than acetic acid and propanoic acid due to the absence of an electron-donating alkyl group. The acidity of carboxylic acids is influenced by the stability of their conjugate bases. Formic acid’s conjugate base (formate ion) is more stable than that of acetic acid (acetate ion) because the negative charge on the formate ion is less destabilized by resonance compared to the acetate ion, which has an alkyl group that can push electron density toward the carboxylate. Benzoic acid, while it has a resonance-stabilized conjugate base, is influenced by the electron-donating effects of the phenyl group, making it less acidic than formic acid.
Question 2
What is the main product formed when butanoic acid reacts with an excess of ethanol in the presence of sulfuric acid?
A) Butanoate
B) Ethyl butanoate
C) Butanol
D) Butyl acetate
Answer: B) Ethyl butanoate
Explanation: The reaction between butanoic acid and ethanol in the presence of sulfuric acid is an esterification reaction, specifically Fischer esterification. The sulfuric acid acts as a catalyst to promote the reaction between the carboxylic acid (butanoic acid) and the alcohol (ethanol). The resulting product, ethyl butanoate, is an ester formed from the hydroxyl group of the acid and the hydrogen from the alcohol. The excess ethanol ensures a higher yield of the ester product by driving the reaction towards the formation of ethyl butanoate and releasing water as a byproduct.
Question 3
Which statement about carboxylic acids is FALSE?
A) Carboxylic acids can form hydrogen bonds with water.
B) The boiling point of carboxylic acids is lower than that of alcohols.
C) Carboxylic acids can undergo decarboxylation.
D) Carboxylic acids are generally more acidic than alcohols.
Answer: B) The boiling point of carboxylic acids is lower than that of alcohols.
Explanation: This statement is false because carboxylic acids typically have higher boiling points than alcohols due to their ability to form strong hydrogen bonds, both with each other and with water. The presence of the carboxyl group (-COOH) allows for more extensive hydrogen bonding compared to the hydroxyl group (-OH) in alcohols. This leads to increased boiling points for carboxylic acids relative to alcohols of similar molecular weight. The other statements are true: carboxylic acids do form hydrogen bonds with water, they can undergo decarboxylation (loss of CO₂), and they are more acidic than alcohols due to the stability of their conjugate bases.