Covalent bonds

- Notes
Covalent bonds are the chemical bonds formed when atoms share pairs of electrons to achieve a full outer electron shell. Understanding covalent bonding is crucial for the MCAT, as it helps explain molecular structure, polarity, and chemical reactivity, all of which are fundamental to biological systems and reactions.
Learning Objectives
In studying covalent bonds for the MCAT, you should understand the principles of electron sharing between atoms, be able to draw Lewis structures, and differentiate between single, double, and triple bonds. You must also recognize the importance of bond polarity and electronegativity in determining molecular properties. Additionally, mastering concepts like bond length, bond energy, and hybridization is essential for predicting molecular geometry and behavior in reactions.
What is a Covalent Bond?
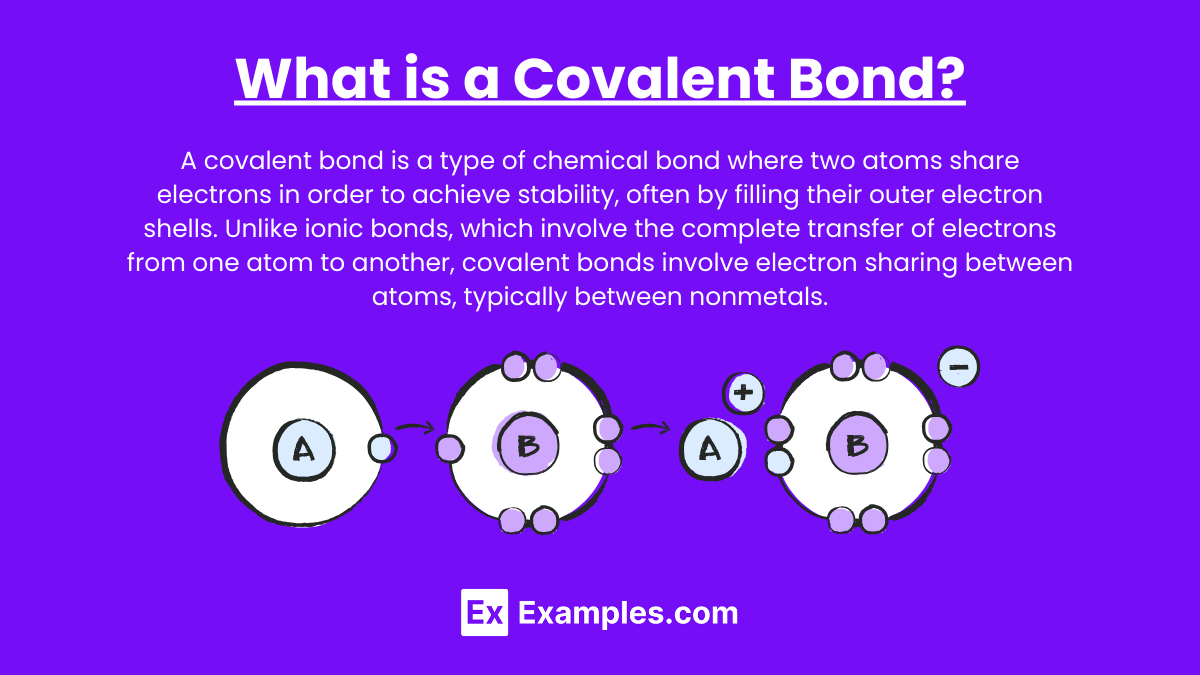
A covalent bond is a type of chemical bond where two atoms share electrons in order to achieve stability, often by filling their outer electron shells. Unlike ionic bonds, which involve the complete transfer of electrons from one atom to another, covalent bonds involve electron sharing between atoms, typically between nonmetals.
Types of Covalent Bonds
- Single Bonds: These involve one pair of shared electrons between two atoms. Single bonds are the longest and weakest type of covalent bonds. They are typically represented as a single line (−) between atoms in a Lewis structure.
- Double Bonds: These occur when two pairs of electrons are shared between two atoms. Double bonds are shorter and stronger than single bonds and are represented by two lines (=) in a Lewis structure.
- Triple Bonds: These involve the sharing of three pairs of electrons between two atoms. Triple bonds are the shortest and strongest type of covalent bonds, represented by three lines (≡) in a Lewis structure.
Bond Polarity and Electronegativity
Covalent bonds can be either nonpolar or polar depending on the difference in electronegativity between the atoms involved:
- Nonpolar Covalent Bonds: If two atoms have similar electronegativity, the electrons are shared equally, and the bond is nonpolar. Examples include bonds between identical atoms, such as H−H or O−O.
- Polar Covalent Bonds: When one atom has a higher electronegativity than the other, the electrons are shared unequally, creating a polar bond. This results in a partial positive charge on one atom and a partial negative charge on the other, as seen in molecules like H−Cl or H₂O.
Bond Length and Bond Energy
- Bond Length: The distance between the nuclei of two bonded atoms. Single bonds are the longest, followed by double bonds, and then triple bonds, which are the shortest.
- Bond Energy: The energy required to break a covalent bond. The stronger the bond (i.e., double or triple bonds), the higher the bond energy.
Hybridization
Hybridization describes how atomic orbitals combine to form new hybrid orbitals for bonding:
- sp Hybridization: Linear geometry with 180° bond angles, as seen in molecules like acetylene (C≡C).
- sp² Hybridization: Trigonal planar geometry with 120° bond angles, common in molecules with double bonds, like ethene (C=C).
- sp³ Hybridization: Tetrahedral geometry with 109.5° bond angles, typical for molecules with single bonds, such as methane (CH₄).
Examples
Example 1: Methane ( $ \text{CH}_4 $ )
Methane has four single covalent bonds between the central carbon atom and four hydrogen atoms. The carbon atom undergoes sp³ hybridization, resulting in a tetrahedral geometry with bond angles of 109.5°. Each bond is formed by the sharing of one pair of electrons.
Example 2: Oxygen ( $ \text{O}_2 $ )
Oxygen molecules consist of a double bond between two oxygen atoms, sharing two pairs of electrons. The bond is shorter and stronger than a single bond, and each oxygen atom satisfies the octet rule by sharing electrons.
Example 3: Nitrogen ( $ \text{N}_2 $ )
Nitrogen molecules form a triple bond between two nitrogen atoms, sharing three pairs of electrons. This bond is very strong and short, making nitrogen gas (N₂) relatively inert under normal conditions.
Example 4: Water ( $ \text{H}_2\text{O} $ )
Water has polar covalent bonds between oxygen and hydrogen. Oxygen is more electronegative than hydrogen, so it pulls the shared electrons closer, creating partial negative and positive charges on oxygen and hydrogen, respectively. Water has a bent shape due to the two lone pairs on oxygen, resulting in bond angles of about 104.5°.
Example 5: Carbon Dioxide ( $ \text{CO}_2 $ )
Carbon dioxide consists of two double bonds between the central carbon atom and each oxygen atom. The molecule is linear due to the sp hybridization of the carbon atom, with bond angles of 180°. Both oxygen atoms satisfy the octet rule, and the double bonds make the structure relatively strong and stable.
Practice Questions
Question 1:
Which of the following molecules contains a triple bond?
A) $ \text{CH}_4 $
B) $ \text{O}_2 $
C) $ \text{N}_2 $
D) $ \text{H}_2O $
Answer: C) $ \text{N}_2 $
Explanation: Nitrogen (N₂) has a triple bond between the two nitrogen atoms, sharing three pairs of electrons. The other molecules have either single or double bonds.
Question 2:
Which of the following bonds is the most polar?
A) C−C
B) C−H
C) H−F
D) O−O
Answer: C) H−F
Explanation: Fluorine is the most electronegative element, so the bond between hydrogen and fluorine is highly polar, with fluorine pulling the electrons strongly towards itself.
Question 3:
What is the hybridization of the carbon atom in ethene ( $ \text{C}_2\text{H}_4 $ )?
A) sp
B) sp²
C) sp³
D) No hybridization
Answer: B) sp²
Explanation: The carbon atoms in ethene (C₂H₄) are involved in a double bond and have sp² hybridization, giving the molecule a trigonal planar geometry with 120° bond angles.