Dot structures

- Notes
Lewis dot structures represent the arrangement of valence electrons in molecules and ions, highlighting bonding and lone pairs. These structures help predict molecular geometry, reactivity, and stability. By mastering Lewis structures, including exceptions to the octet rule and resonance, you’ll strengthen your understanding of molecular interactions for the MCAT.
Learning Objectives
In studying dot structures for the MCAT, you should understand how to draw Lewis dot structures, account for valence electrons, and recognize bonding and lone pairs. Learn to apply the octet rule and identify exceptions, such as incomplete or expanded octets. Additionally, you should be able to predict resonance structures and calculate formal charges, ensuring you can assess the stability of molecules and ions.
What are Lewis Dot Structures?
Lewis dot structures, also called electron dot structures, are diagrams that represent the valence electrons of atoms within a molecule. These structures show how atoms share electrons to form chemical bonds and help in predicting the geometry, reactivity, and properties of molecules.
Bonding and Lone Pairs
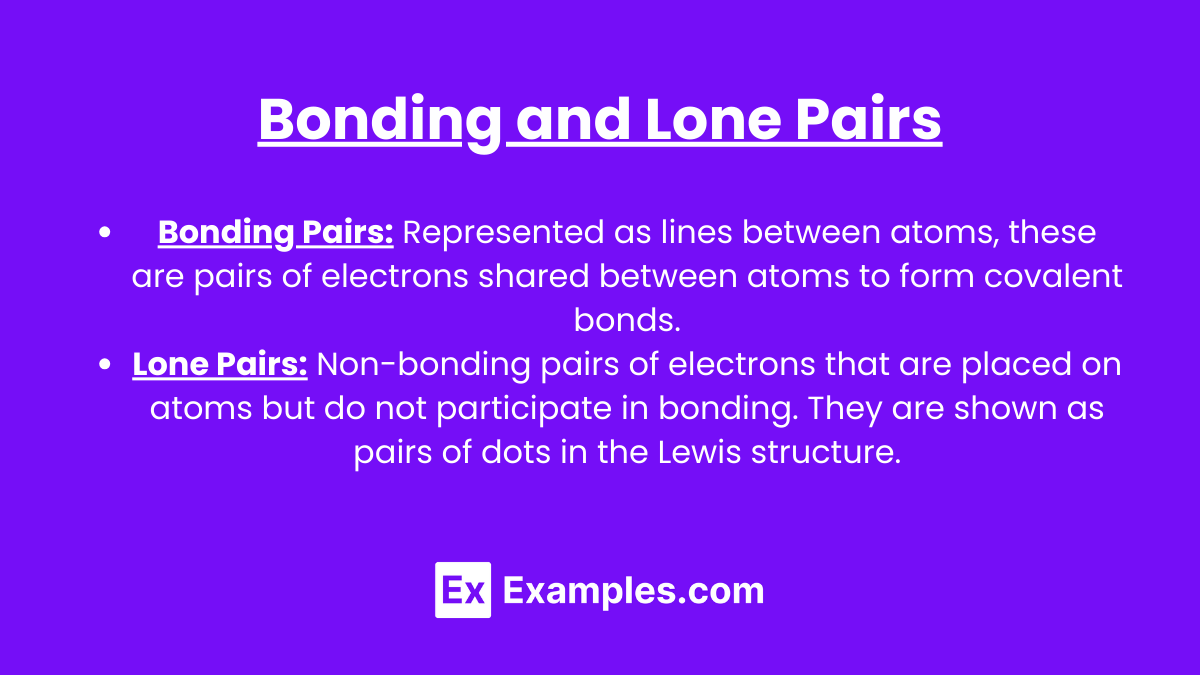
Bonding Pairs: Represented as lines between atoms, these are pairs of electrons shared between atoms to form covalent bonds.
Lone Pairs: Non-bonding pairs of electrons that are placed on atoms but do not participate in bonding. They are shown as pairs of dots in the Lewis structure.
Exceptions to the Octet Rule
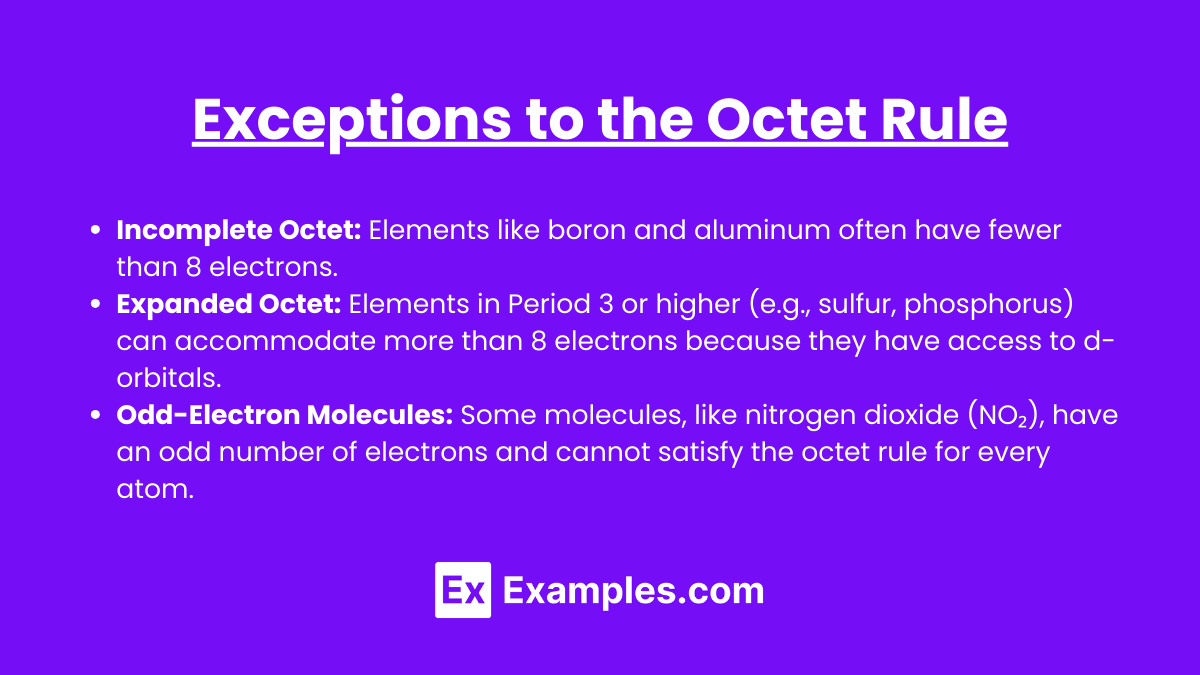
While the octet rule applies to many molecules, there are important exceptions:
- Incomplete Octet: Elements like boron and aluminum often have fewer than 8 electrons.
- Expanded Octet: Elements in Period 3 or higher (e.g., sulfur, phosphorus) can accommodate more than 8 electrons because they have access to d-orbitals.
- Odd-Electron Molecules: Some molecules, like nitrogen dioxide (NO₂), have an odd number of electrons and cannot satisfy the octet rule for every atom.
Resonance Structures
Some molecules have more than one valid Lewis structure. These are called resonance structures, and they represent the delocalization of electrons. For example, in ozone (O₃), the double bond can be placed between different oxygen atoms, creating multiple resonance forms. The true structure is a hybrid of all the resonance structures.
Examples
Example 1: Methane ( $ \text{CH}_4 $ )
Methane has four single bonds between the central carbon atom and four hydrogen atoms. The Lewis structure is: H−C−H
Each hydrogen has 2 electrons, and carbon has a full octet (8 electrons).
Example 2: Phosphorus Pentachloride ( $ \text{PCl}_5 $ )
Phosphorus pentachloride is an example of an expanded octet, where phosphorus is surrounded by 10 electrons. The Lewis structure is: Cl−P−Cl
Phosphorus uses d-orbitals to accommodate more than 8 electrons.
Example 3: Formaldehyde ( $ \text{CH}_2\text{O} $ )
Formaldehyde has a double bond between carbon and oxygen, and single bonds between carbon and two hydrogen atoms. The Lewis structure is: H−C=O ∣ H
Oxygen has 2 lone pairs and satisfies the octet rule, as does carbon.
Example 4: Nitrogen Dioxide ( $ \text{NO}_2 $ )
Nitrogen dioxide has an odd number of electrons, making it a free radical. The structure has resonance:
O−N=O or O=N−O
Nitrogen has an incomplete octet.
Example 5: Chlorate Ion ( $ \text{ClO}_3^- $ )
The chlorate ion has a resonance structure where the double bond between chlorine and oxygen can shift. The Lewis structure is: O = Cl−O– O–
The resonance stabilizes the ion, with chlorine in an expanded octet.
Practice Questions
Question 1:
Which of the following molecules follows the octet rule?
A) $ \text{PCl}_5 $
B) $ \text{BF}_3 $
C) $ \text{CH}_4 $
D) $ \text{NO}_2 $
Answer:
C) $ \text{CH}_4 $
Explanation:
Methane ( $ \text{CH}_4 $ ) has carbon surrounded by 8 electrons, fulfilling the octet rule. In contrast, $ \text{PCl}_5 $ has an expanded octet, $ \text{BF}_3 $ has an incomplete octet, and $ \text{NO}_2 $ is a free radical with an odd number of electrons.
Question 2:
Which molecule or ion exhibits resonance?
A) $ \text{HCl} $
B) $ \text{CH}_4 $
C) $ \text{O}_3 $
D) $ \text{NH}_3 $
Answer:
C) $ \text{O}_3 $
Explanation:
Ozone ( $ \text{O}_3 $ ) has resonance because the double bond can be placed between different oxygen atoms, creating multiple valid Lewis structures. The others do not exhibit resonance.
Question 3:
How many lone pairs of electrons are present on the oxygen atoms in the chlorate ion ( $ \text{ClO}_3^- $ )?
A) 6
B) 9
C) 12
D) 3
Answer:
C) 12
Explanation:
Each of the three oxygen atoms in $ \text{ClO}_3^- $ has 2 lone pairs, for a total of 6 lone pairs (12 electrons).