The electronic structure of atoms is fundamental to understanding chemical behavior, laying the foundation for atomic theory and quantum mechanics. On the MCAT, you’ll explore electron configurations, quantum numbers, and orbital shapes. Understanding the periodic trends, such as electronegativity and ionization energy, is essential, as these concepts play a key role in mastering general chemistry and physical science sections of the exam.
Learning Objectives
In studying "Electronic Structure" for the MCAT, you should learn to understand how electrons are arranged within atoms, focusing on energy levels, subshells, and orbitals. Differentiate between the principal quantum numbers and how they determine the energy and distribution of electrons. Analyze electron configurations, including the use of Hund’s rule, the Pauli exclusion principle, and the Aufbau principle, to predict how electrons populate orbitals. Evaluate the relationship between electronic structure and chemical properties, such as ionization energy and atomic radii. Additionally, explore how excited states, electron transitions, and the emission or absorption of energy are fundamental to atomic spectroscopy.
Understanding the Arrangement of Electrons in Atoms
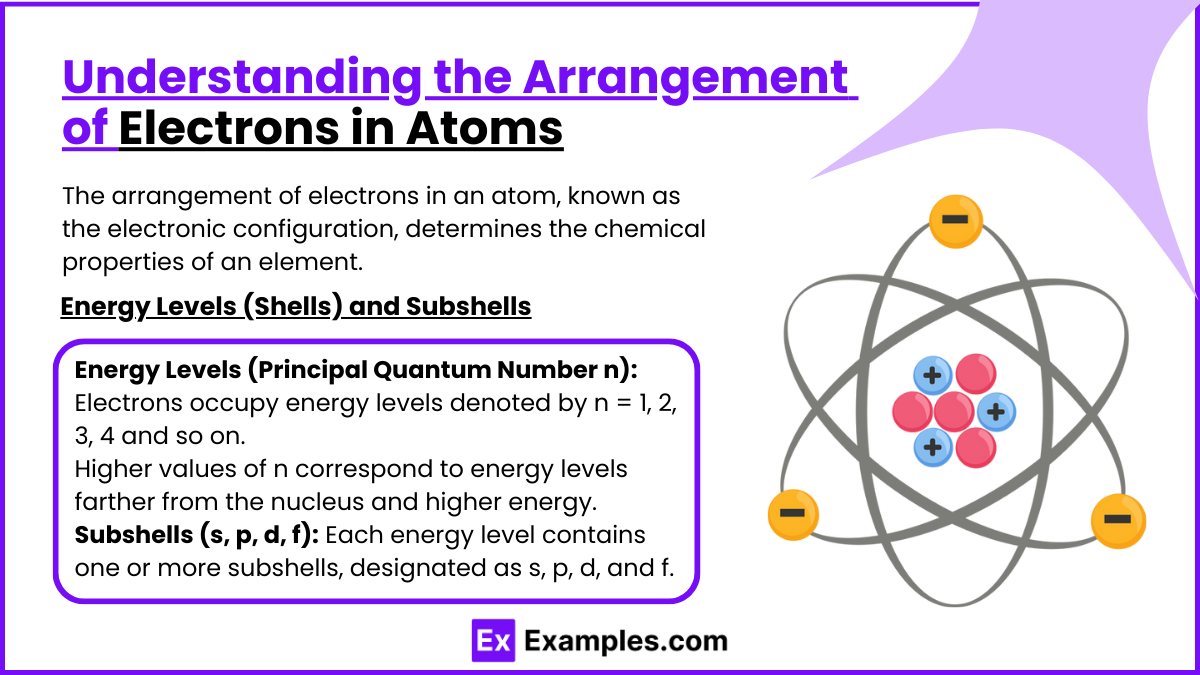
The arrangement of electrons in an atom, known as the electronic configuration, determines the chemical properties of an element. Electrons are distributed around the nucleus in energy levels (shells), subshells, and orbitals, following specific principles and rules. This configuration explains how atoms interact, form bonds, and participate in chemical reactions.
Energy Levels (Shells) and Subshells
Energy Levels (Principal Quantum Number n):
Electrons occupy energy levels denoted by n = 1, 2, 3, 4 and so on.
Higher values of n correspond to energy levels farther from the nucleus and higher energy.
Subshells (s, p, d, f):
Each energy level contains one or more subshells, designated as s, p, d, and f.
These subshells contain orbitals that hold electrons:
s-subshell: 1 orbital (can hold 2 electrons).
p-subshell: 3 orbitals (can hold 6 electrons).
d-subshell: 5 orbitals (can hold 10 electrons).
f-subshell: 7 orbitals (can hold 14 electrons).
Rules for Electron Arrangement
Aufbau Principle:
Electrons fill orbitals in order of increasing energy. Lower-energy orbitals fill before higher-energy ones.
The typical filling order is: 1s→2s→2p→3s→3p→4s→3d→4p→5s→4d→5p→6s→4f→5d
For example, carbon (Z = 6) has the configuration: 1s2 2s2 2p2.
Pauli Exclusion Principle:
No two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold at most two electrons with opposite spins.
Hund's Rule:
When electrons fill degenerate orbitals (orbitals of the same energy), one electron enters each orbital until all are half-filled, with parallel spins. This minimizes electron repulsion.
Electron Configurations of Elements
Hydrogen (Z = 1): 1s1
Helium (Z = 2): 1s2
Oxygen (Z = 8): 1s2 2s2 2p4
Sodium (Z = 11): 1s2 2s2 2p6 3s1
Valence Electrons and Chemical Reactivity
Valence Electrons: The electrons in the outermost shell (highest nnn) are called valence electrons. These electrons determine the chemical reactivity and bonding behavior of an element.
Example: Sodium (Z = 11) has 1 valence electron in the 3s orbital, which makes it highly reactive and prone to losing that electron to form a Na+ ion.
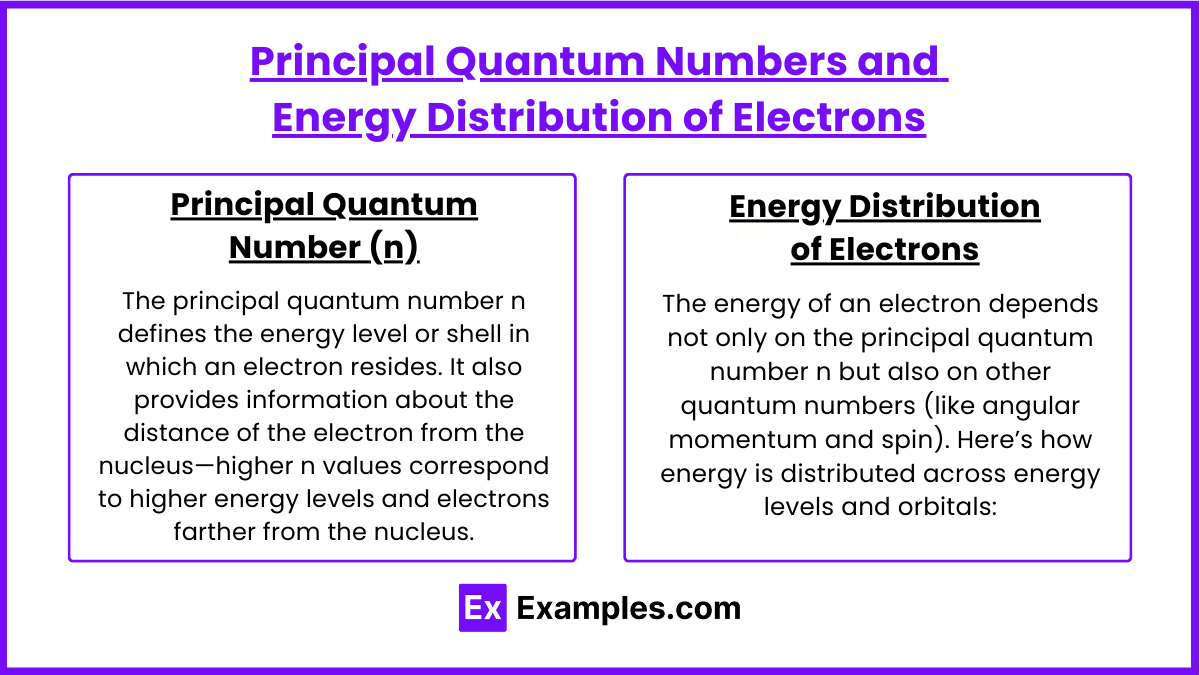
Principal Quantum Numbers and Energy Distribution of Electrons
The principal quantum number and other quantum numbers play a fundamental role in understanding how electrons are arranged within an atom. These quantum numbers determine the energy levels, shape, and spatial orientation of electron clouds (orbitals) around the nucleus. The energy distribution of electrons depends on their quantum state, and this distribution influences the chemical properties of the atom.
Principal Quantum Number (n)
The principal quantum number n defines the energy level or shell in which an electron resides. It also provides information about the distance of the electron from the nucleus—higher n values correspond to higher energy levels and electrons farther from the nucleus.
Possible values: n = 1, 2, 3, 4,… (positive integers).
Energy Levels: Electrons in higher nnn shells have more energy and are less tightly bound to the nucleus.
n = 1 is the first (innermost) energy level (K-shell).
n = 2 is the second energy level (L-shell), and so on.
Example: For hydrogen, the electron in the ground state is in the n = 1 energy level.
Energy Distribution of Electrons
The energy of an electron depends not only on the principal quantum number n but also on other quantum numbers (like angular momentum and spin). Here’s how energy is distributed across energy levels and orbitals:
Shells and Subshells:
Each energy level n contains subshells denoted by the angular momentum quantum number (l).
l = 0 corresponds to an s-orbital (spherical).
l = 1 corresponds to a p-orbital (dumbbell-shaped).
l = 2 corresponds to a d-orbital.
l = 3 corresponds to an f-orbital.
Energy Hierarchy:
In general, the energy of an orbital increases with increasing n. However, within the same n-level, the subshells have different energy:
s<p<d<f (for the same n).
For example: In the second energy level (n = 2), the 2s orbital has lower energy than the 2p orbitals.
Electron Filling Order:
Electrons fill orbitals in the order of increasing energy, as described by the Aufbau principle: 1s→2s→2p→3s→3p→4s→3d→4p→5s→4d
This filling order balances between increasing nnn and the subshell energy hierarchy.
Quantum Numbers and Energy Levels
Principal Quantum Number (n): Determines the shell/energy level and electron’s energy.
Angular Momentum Quantum Number (l): Determines the subshell (s, p, d, f) and orbital shape.
Magnetic Quantum Number (ml): Determines the orientation of the orbital.
Spin Quantum Number (ms): Describes the electron’s spin (+1/2 or −1/2).
Electron Configurations and Principles of Orbital Filling
Electron configurations describe the arrangement of electrons in an atom's orbitals, providing insight into its chemical properties. This arrangement follows specific rules and principles, ensuring that electrons occupy the most stable and lowest energy levels available.
Key Principles of Orbital Filling
Aufbau Principle (German for "building up"):
Electrons fill orbitals in the order of increasing energy, starting from the lowest energy level (1s) to higher levels.
The order of filling is determined by the increasing energy of the orbitals, given approximately by: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p
Notice that 4s is filled before 3d due to its slightly lower energy.
Pauli Exclusion Principle:
No two electrons in an atom can have the same set of four quantum numbers.
As a result, an orbital can hold a maximum of two electrons with opposite spins.
Hund's Rule:
When electrons occupy orbitals of the same energy (degenerate orbitals), one electron enters each orbital with the same spin before any orbital gets a second electron.
This minimizes electron-electron repulsion and stabilizes the atom.
Orbital Types and Their Capacities
s orbital: Holds a maximum of 2 electrons (1 orbital)
p orbitals: Hold a maximum of 6 electrons (3 orbitals)
d orbitals: Hold a maximum of 10 electrons (5 orbitals)
f orbitals: Hold a maximum of 14 electrons (7 orbitals)
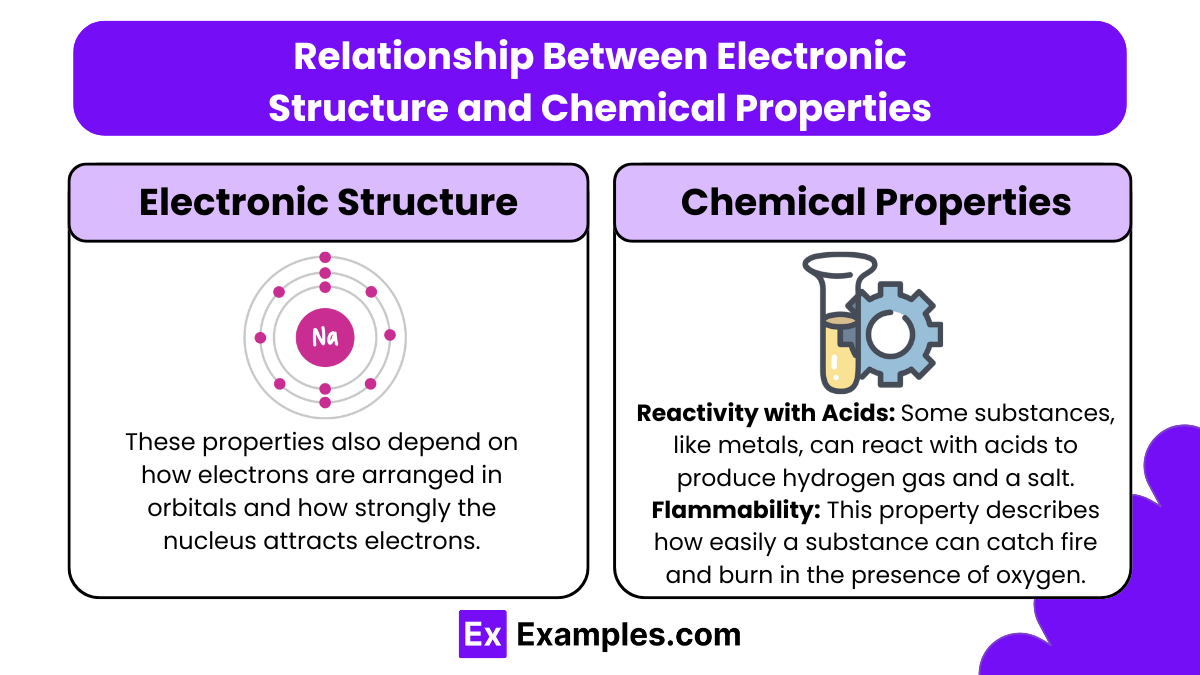
Relationship Between Electronic Structure and Chemical Properties
The arrangement of electrons influences many chemical properties, such as:
Ionization Energy: The energy required to remove an electron from an atom. Atoms with a stable electronic configuration (such as noble gases) have high ionization energies.
Atomic Radius: The size of an atom, which decreases across a period as electrons are added to the same energy level, increasing the nuclear attraction. Atomic radius increases down a group as additional energy levels are added.
Electron Affinity and Electronegativity: These properties also depend on how electrons are arranged in orbitals and how strongly the nucleus attracts electrons.
1. Valence Electrons and Reactivity
Valence electrons are the outermost electrons that participate in chemical reactions.
Same Group = Similar Properties: Elements in the same group of the periodic table (like the alkali metals) have the same number of valence electrons and react similarly.
Example: Sodium (Na) and potassium (K) both have 1 valence electron and react quickly with water.
2. Ionization Energy (How Easily an Atom Loses Electrons)
Low ionization energy means an atom loses electrons easily, making it more reactive (like sodium).
High ionization energy means it’s harder to remove electrons, so the atom is less likely to lose them.
Example: Sodium loses one electron easily, but fluorine holds its electrons tightly.
3. Electronegativity (How Strongly an Atom Attracts Electrons)
High electronegativity (like fluorine) pulls electrons toward it, making it good at forming bonds.
Low electronegativity (like sodium) easily gives away electrons.
Example: Sodium and chlorine bond to form NaCl (salt) because sodium gives up an electron, and chlorine takes it.
4. Atomic Size and Reactivity
Larger atoms (like potassium) lose electrons easily because their outermost electrons are far from the nucleus.
Smaller atoms (like fluorine) attract electrons better, making them more reactive with other elements.
5. Stable Electron Configurations and the Octet Rule
Noble gases (like neon) have full outer shells, so they don’t react much.
Most elements try to get 8 valence electrons (a full outer shell) by gaining, losing, or sharing electrons.
Example: Sodium gives up 1 electron to become Na⁺, achieving the same electron structure as neon.
Examples
Example 1: Hydrogen Atom
The electronic structure of the hydrogen atom consists of a single electron orbiting a single proton. This simple arrangement allows for the study of fundamental quantum mechanics and atomic behavior. The electron occupies specific energy levels, or orbitals, with distinct quantized states. The simplicity of hydrogen's electronic structure serves as a model for understanding more complex atoms.
Example 2: Electron Configuration in Elements
The electronic structure of elements is described by their electron configurations, which indicate the distribution of electrons among atomic orbitals. For instance, the electron configuration of carbon is 1s2 2s2 2p2, meaning it has two electrons in the first energy level and four in the second. Understanding electron configurations helps predict an element's chemical properties, reactivity, and bonding behavior.
Example 3: Valence Electrons and Chemical Bonds
The concept of valence electrons, which are the electrons in the outermost shell of an atom, is crucial for understanding chemical bonding. For example, in sodium (Na), the electronic structure is 1s2 2s2 2p6 3s1, indicating that sodium has one valence electron. This single valence electron is easily lost during chemical reactions, leading to the formation of ionic bonds, particularly with nonmetals like chlorine, which has a tendency to gain electrons.
Example 4: Transition Metals and d-Orbitals
Transition metals possess complex electronic structures that include d-orbitals. For example, in iron (Fe), the electron configuration is 1s2 2s2 2p6 3s23p63d64s2. The presence of partially filled d-orbitals allows transition metals to exhibit unique properties such as variable oxidation states, colorful compounds, and magnetic behavior. This complexity in electronic structure is significant for understanding the chemistry and applications of transition metals.
Example 5: Ionic and Covalent Compounds
The electronic structure plays a vital role in the formation of ionic and covalent compounds. For instance, in sodium chloride (NaCl), sodium loses its single valence electron to achieve a stable electron configuration, while chlorine gains an electron to complete its valence shell. This transfer of electrons leads to the formation of oppositely charged ions, which are held together by electrostatic forces. In contrast, in covalent compounds like water (H₂O), atoms share electrons to achieve stable electronic structures, demonstrating how electronic arrangements dictate the type of chemical bonding.
Practice Questions
Question 1
What does the electron configuration of an atom represent?
A) The total number of protons in the nucleus.
B) The arrangement of electrons in atomic orbitals.
C) The number of neutrons present in the nucleus.
D) The mass of the atom.
Correct Answer: B) The arrangement of electrons in atomic orbitals.
Explanation: Electron configuration describes how electrons are distributed among the various atomic orbitals of an atom. It provides insights into the chemical behavior of the atom, including its bonding capabilities and reactivity. The configuration specifies the energy levels and sublevels that electrons occupy but does not provide information about protons or neutrons or the overall mass of the atom.
Question 2
Which of the following statements is true regarding valence electrons?
A) They are always found in the innermost shell.
B) They determine the chemical properties of an element.
C) They are not involved in chemical bonding.
D) They are the same as core electrons.
Correct Answer: B) They determine the chemical properties of an element.
Explanation: Valence electrons are the electrons in the outermost shell of an atom and play a crucial role in chemical bonding and reactivity. The number of valence electrons determines how an element interacts with others, influencing its chemical properties and the types of bonds it can form. Options A, C, and D are incorrect; valence electrons are in the outer shell, are involved in bonding, and are distinct from core electrons, which are located in inner shells.
Question 3
Which principle states that no two electrons in an atom can have the same set of four quantum numbers?
A) Hund's Rule
B) Pauli Exclusion Principle
C) Aufbau Principle
D) Dalton's Law
Correct Answer: B) Pauli Exclusion Principle.
Explanation: The Pauli Exclusion Principle states that no two electrons in the same atom can occupy the same quantum state simultaneously, meaning they cannot have identical sets of quantum numbers. This principle is fundamental in explaining the structure of electron shells and subshells, ensuring that electrons are arranged in a way that maximizes the unique states available to them. Hund's Rule and the Aufbau Principle pertain to how electrons are filled into orbitals, while Dalton's Law is unrelated to atomic structure.