Enzyme kinetics

- Notes
Enzyme kinetics explores how enzymes accelerate chemical reactions, focusing on reaction rates and factors that influence them. Key concepts include the Michaelis-Menten equation, enzyme-substrate interactions, and types of inhibition. Understanding these principles is crucial for the MCAT, as they illustrate enzyme functionality in biological processes and inform pharmacological applications like drug targeting and metabolic regulation.
Learning Objectives
In studying “Enzyme Kinetics” for the MCAT, you should learn to understand how enzymes facilitate chemical reactions and the factors that influence their rates. Analyze key concepts like the Michaelis-Menten equation, Vmax, Km, and catalytic efficiency. Evaluate how substrate concentration, inhibitors, and environmental conditions (pH, temperature) impact enzyme activity. Additionally, explore types of enzyme inhibition (competitive, noncompetitive, uncompetitive) and how these affect reaction rates. Apply this knowledge to MCAT practice passages to interpret kinetic data, graphs, and experimental results related to enzyme function and regulation.
1. Basic Principles of Enzyme Kinetics
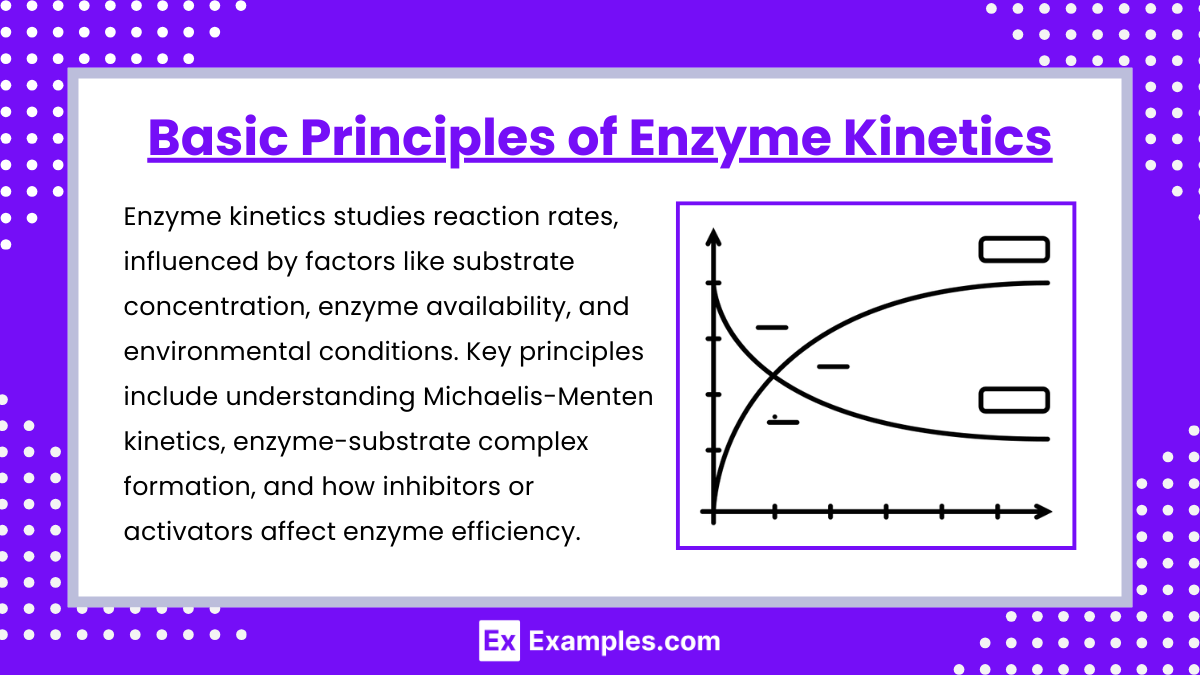
- Enzymes are biological catalysts that increase the rate of reactions by lowering the activation energy without being consumed in the process.
- Active sites are specific regions on enzymes where substrates bind, forming the enzyme-substrate (ES) complex.
- The rate of an enzyme-catalyzed reaction depends on substrate concentration, enzyme concentration, temperature, pH, and the presence of inhibitors or activators.
2. Michaelis-Menten Kinetics
- Describes the relationship between substrate concentration [S] and reaction rate vvv for enzyme-catalyzed reactions.
- The Michaelis-Menten equation: v =
where:- Vₘₐₓ: Maximum reaction rate achieved by the system, at saturation substrate concentration.
- Kₘ: Michaelis constant, the substrate concentration at which the reaction rate is half of Vₘₐₓ. It indicates enzyme affinity for the substrate—lower Kₘ values imply higher affinity.
3. Enzyme Inhibition
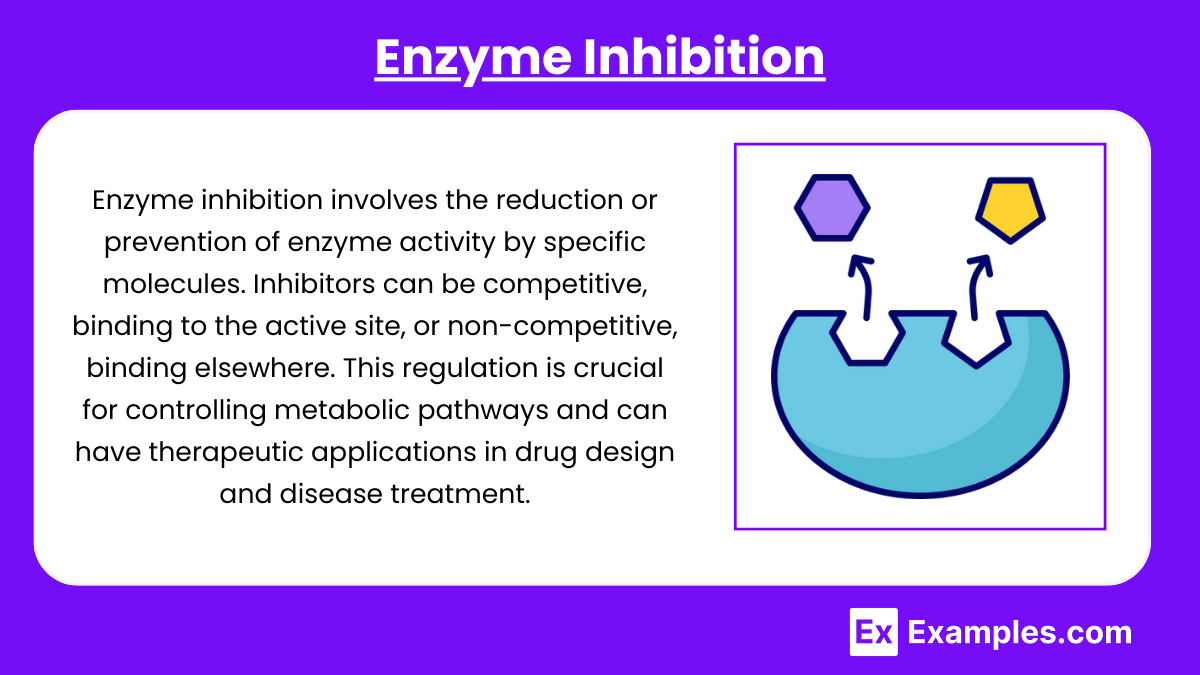
- Competitive Inhibition: Inhibitors compete with the substrate for the active site. This increases Kₘ without affecting Vₘₐₓ.
- Non-competitive Inhibition: Inhibitors bind to an allosteric site (not the active site), reducing Vₘₐₓ without changing Kₘ.
- Uncompetitive Inhibition: Inhibitors bind only to the ES complex, decreasing both Kₘ and Vₘₐₓ.
4. Lineweaver-Burk Plot
- This is a double reciprocal plot (1/v vs. 1/[S]) used to linearize the Michaelis-Menten equation, facilitating the determination of Kₘ and Vₘₐₓ.
- The slope of the Lineweaver-Burk plot equals Kₘ/Vₘₐₓ, the y-intercept is 1/Vₘₐₓ, and the x-intercept is −1/Kₘ.
5. Factors Influencing Enzyme Activity
- Temperature: Each enzyme has an optimal temperature; deviations can lead to denaturation or reduced activity.
- pH: Enzymes have an optimal pH range, and deviations can affect ionization of active site residues, altering activity.
- Enzyme and Substrate Concentration: Higher concentrations generally increase reaction rates, but only up to a saturation point where all enzyme molecules are engaged.
Examples
Example 1: Alcohol Dehydrogenase and Ethanol Metabolism
Alcohol dehydrogenase converts ethanol to acetaldehyde, with a specific KmK_mKm reflecting the enzyme’s affinity for ethanol. This reaction illustrates how enzyme saturation affects alcohol processing in the liver.
Example 2: Lactase and Lactose Digestion
Lactase breaks down lactose into glucose and galactose. Variations in lactase activity influence lactose tolerance, demonstrating how enzyme kinetics govern the effectiveness of carbohydrate digestion.
Example 3: Hexokinase in Glycolysis
Hexokinase phosphorylates glucose, the first step in glycolysis. Its low KmK_mKm for glucose ensures rapid glucose utilization in cells, even at low concentrations, highlighting substrate affinity’s impact on metabolic pathways.
Example 4: Angiotensin-Converting Enzyme (ACE) and Blood Pressure
ACE converts angiotensin I to angiotensin II, regulating blood pressure. ACE inhibitors reduce this conversion, showcasing competitive inhibition’s role in therapeutic enzyme targeting.
Example 5: Pepsin in Protein Digestion
Pepsin hydrolyzes proteins in the stomach. Its optimal activity at low pH demonstrates how environmental conditions, such as acidity, affect enzyme kinetics and substrate binding efficiency.
Practice Questions
Question 1:
Which of the following statements about Kₘ in enzyme kinetics is correct?
A) It represents maximum reaction rate.
B) It indicates enzyme-substrate binding affinity.
C) It reflects enzyme concentration.
D) It remains constant regardless of inhibitors.
Answer: B) It indicates enzyme-substrate binding affinity.
Explanation: Kₘ is the Michaelis constant, indicating enzyme affinity for a substrate. A lower Kₘ means higher affinity. It is not related to reaction rate or enzyme concentration and can change with competitive inhibition.
Question 2:
Which type of inhibition decreases both Vₘₐₓ and Kₘ?
A) Competitive
B) Non-competitive
C) Uncompetitive
D) Allosteric
Answer: C) Uncompetitive
Explanation: Uncompetitive inhibition binds only to the enzyme-substrate complex, decreasing both Vₘₐₓ and Kₘ. It differs from non-competitive inhibition, which affects Vₘₐₓ but not Kₘ.
Question 3:
What effect does competitive inhibition have on KmK_mKm and VmaxV_{\text{max}}Vmax?
A) Increases Kₘ, Vₘₐₓ constant
B) Decreases Kₘ, Vₘₐₓ decreases
C) Kₘ and Vₘₐₓ remain unchanged
D) Decreases Kₘ, Vₘₐₓ unchanged
Answer: A) Increases Kₘ, Vₘₐₓ constant
Explanation: Competitive inhibition increases Kₘ as it reduces enzyme affinity for the substrate. However, it does not affect Vₘₐₓ, as adding more substrate can overcome the inhibitor’s effect.
Enzyme kinetics explores how enzymes accelerate chemical reactions, focusing on reaction rates and factors that influence them. Key concepts include the Michaelis-Menten equation, enzyme-substrate interactions, and types of inhibition. Understanding these principles is crucial for the MCAT, as they illustrate enzyme functionality in biological processes and inform pharmacological applications like drug targeting and metabolic regulation.
Learning Objectives
In studying "Enzyme Kinetics" for the MCAT, you should learn to understand how enzymes facilitate chemical reactions and the factors that influence their rates. Analyze key concepts like the Michaelis-Menten equation, Vmax, Km, and catalytic efficiency. Evaluate how substrate concentration, inhibitors, and environmental conditions (pH, temperature) impact enzyme activity. Additionally, explore types of enzyme inhibition (competitive, noncompetitive, uncompetitive) and how these affect reaction rates. Apply this knowledge to MCAT practice passages to interpret kinetic data, graphs, and experimental results related to enzyme function and regulation.
1. Basic Principles of Enzyme Kinetics

Enzymes are biological catalysts that increase the rate of reactions by lowering the activation energy without being consumed in the process.
Active sites are specific regions on enzymes where substrates bind, forming the enzyme-substrate (ES) complex.
The rate of an enzyme-catalyzed reaction depends on substrate concentration, enzyme concentration, temperature, pH, and the presence of inhibitors or activators.
2. Michaelis-Menten Kinetics

Describes the relationship between substrate concentration [S] and reaction rate vvv for enzyme-catalyzed reactions.
The Michaelis-Menten equation: v = Km+[S]Vmax[S]$\frac{{V_{\text{max}} [S]}}{{K_m + [S]}}$
where:Vₘₐₓ: Maximum reaction rate achieved by the system, at saturation substrate concentration.
Kₘ: Michaelis constant, the substrate concentration at which the reaction rate is half of Vₘₐₓ. It indicates enzyme affinity for the substrate—lower Kₘ values imply higher affinity.
3. Enzyme Inhibition

Competitive Inhibition: Inhibitors compete with the substrate for the active site. This increases Kₘ without affecting Vₘₐₓ.
Non-competitive Inhibition: Inhibitors bind to an allosteric site (not the active site), reducing Vₘₐₓ without changing Kₘ.
Uncompetitive Inhibition: Inhibitors bind only to the ES complex, decreasing both Kₘ and Vₘₐₓ.
4. Lineweaver-Burk Plot

This is a double reciprocal plot (1/v vs. 1/[S]) used to linearize the Michaelis-Menten equation, facilitating the determination of Kₘ and Vₘₐₓ.
The slope of the Lineweaver-Burk plot equals Kₘ/Vₘₐₓ, the y-intercept is 1/Vₘₐₓ, and the x-intercept is −1/Kₘ.
5. Factors Influencing Enzyme Activity

Temperature: Each enzyme has an optimal temperature; deviations can lead to denaturation or reduced activity.
pH: Enzymes have an optimal pH range, and deviations can affect ionization of active site residues, altering activity.
Enzyme and Substrate Concentration: Higher concentrations generally increase reaction rates, but only up to a saturation point where all enzyme molecules are engaged.
Examples
Example 1: Alcohol Dehydrogenase and Ethanol Metabolism
Alcohol dehydrogenase converts ethanol to acetaldehyde, with a specific KmK_mKm reflecting the enzyme's affinity for ethanol. This reaction illustrates how enzyme saturation affects alcohol processing in the liver.
Example 2: Lactase and Lactose Digestion
Lactase breaks down lactose into glucose and galactose. Variations in lactase activity influence lactose tolerance, demonstrating how enzyme kinetics govern the effectiveness of carbohydrate digestion.
Example 3: Hexokinase in Glycolysis
Hexokinase phosphorylates glucose, the first step in glycolysis. Its low KmK_mKm for glucose ensures rapid glucose utilization in cells, even at low concentrations, highlighting substrate affinity's impact on metabolic pathways.
Example 4: Angiotensin-Converting Enzyme (ACE) and Blood Pressure
ACE converts angiotensin I to angiotensin II, regulating blood pressure. ACE inhibitors reduce this conversion, showcasing competitive inhibition's role in therapeutic enzyme targeting.
Example 5: Pepsin in Protein Digestion
Pepsin hydrolyzes proteins in the stomach. Its optimal activity at low pH demonstrates how environmental conditions, such as acidity, affect enzyme kinetics and substrate binding efficiency.
Practice Questions
Question 1:
Which of the following statements about Kₘ in enzyme kinetics is correct?
A) It represents maximum reaction rate.
B) It indicates enzyme-substrate binding affinity.
C) It reflects enzyme concentration.
D) It remains constant regardless of inhibitors.
Answer: B) It indicates enzyme-substrate binding affinity.
Explanation: Kₘ is the Michaelis constant, indicating enzyme affinity for a substrate. A lower Kₘ means higher affinity. It is not related to reaction rate or enzyme concentration and can change with competitive inhibition.
Question 2:
Which type of inhibition decreases both Vₘₐₓ and Kₘ?
A) Competitive
B) Non-competitive
C) Uncompetitive
D) Allosteric
Answer: C) Uncompetitive
Explanation: Uncompetitive inhibition binds only to the enzyme-substrate complex, decreasing both Vₘₐₓ and Kₘ. It differs from non-competitive inhibition, which affects Vₘₐₓ but not Kₘ.
Question 3:
What effect does competitive inhibition have on KmK_mKm and VmaxV_{\text{max}}Vmax?
A) Increases Kₘ, Vₘₐₓ constant
B) Decreases Kₘ, Vₘₐₓ decreases
C) Kₘ and Vₘₐₓ remain unchanged
D) Decreases Kₘ, Vₘₐₓ unchanged
Answer: A) Increases Kₘ, Vₘₐₓ constant
Explanation: Competitive inhibition increases Kₘ as it reduces enzyme affinity for the substrate. However, it does not affect Vₘₐₓ, as adding more substrate can overcome the inhibitor’s effect.