Enzyme Structure and Function

- Notes
Enzymes are biological catalysts that accelerate chemical reactions by lowering activation energy, crucial for cellular processes. Composed of amino acids, enzymes have specific active sites where substrates bind, facilitating reactions. Factors like temperature, pH, and substrate concentration affect enzyme activity. Understanding enzyme mechanisms, kinetics, and inhibition is essential for mastering biochemical concepts relevant to the MCAT.
Learning Objectives
In studying “Enzyme Structure and Function” for the MCAT, you should learn to understand the basic structure of enzymes, including active sites, cofactors, and how substrate specificity is achieved. Analyze how enzymes act as biological catalysts, affecting reaction rates and lowering activation energy. Evaluate concepts such as enzyme kinetics, including the Michaelis-Menten equation, and factors influencing enzyme activity like pH, temperature, and inhibitors. Additionally, explore how enzymes are regulated through allosteric control and feedback inhibition, and apply these concepts to MCAT practice passages to interpret enzyme-related data and experimental results.
Basic Structure of Enzymes
- Amino Acid Composition: Enzymes are proteins made up of specific sequences of amino acids, which fold into unique three-dimensional shapes.
- Active Site: This is a specialized region where substrates bind and the catalytic reaction occurs. The specificity of the enzyme to its substrate is due to the unique shape and chemical properties of the active site.
- Cofactors and Coenzymes: Many enzymes require additional molecules to be fully functional. Cofactors can be metal ions (like Mg²⁺ or Zn²⁺) or organic molecules called coenzymes (e.g., NAD⁺, FAD).
- Holoenzymes and Apoenzymes: A holoenzyme is a complete enzyme with its cofactor or coenzyme, while an apoenzyme is the protein portion alone, which is inactive.
Enzyme Function and Mechanisms
- Catalysis: Enzymes work by lowering the activation energy of biochemical reactions, thereby increasing reaction rates without being consumed in the process.
- Mechanisms of Action: This includes various models like the Lock-and-Key model, where substrates fit precisely into the active site, and the Induced Fit model, which involves conformational changes to accommodate the substrate.
- Enzyme Kinetics: Understand the Michaelis-Menten equation and key terms like Vmax (maximum velocity), Km (Michaelis constant), and how they relate to enzyme efficiency. These values are crucial for understanding enzyme-substrate affinity.
- Inhibition: Enzymes can be inhibited in multiple ways. Competitive inhibitors bind to the active site, while non-competitive inhibitors bind to an allosteric site, altering the enzyme’s function. Understanding the differences in how each affects Vmax and Km is essential.
Factors Affecting Enzyme Activity
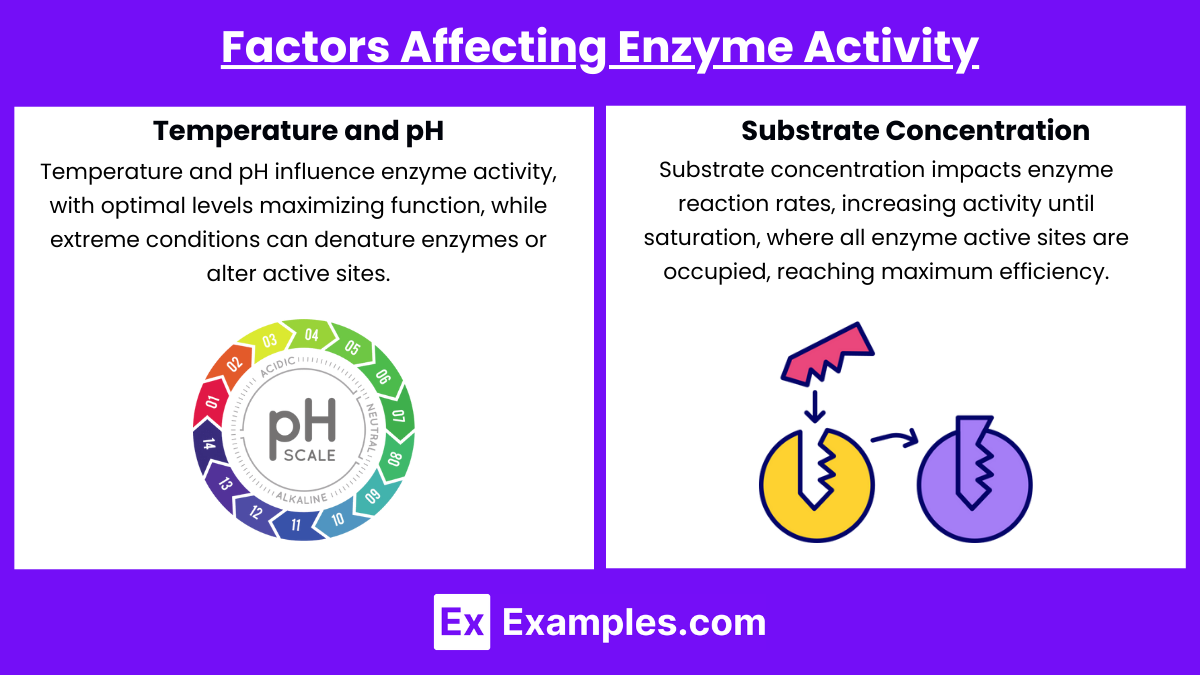
- Temperature and pH: Each enzyme has an optimal temperature and pH at which it functions best. Deviations can lead to denaturation or decreased activity.
- Substrate Concentration: The rate of reaction increases with substrate concentration up to a point, beyond which it plateaus as enzymes become saturated.
Types of Enzymes and Their Functions
- Oxidoreductases: Involved in oxidation-reduction reactions (e.g., dehydrogenases).
- Transferases: Transfer functional groups between molecules (e.g., kinases).
- Hydrolases: Catalyze hydrolysis reactions (e.g., proteases).
- Lyases: Break chemical bonds by means other than hydrolysis or oxidation.
- Isomerases: Catalyze isomerization changes within a molecule.
- Ligases: Join two molecules together with covalent bonds (e.g., DNA ligase).
Examples
Example 1: Amylase
Found in saliva, it catalyzes the breakdown of starch into sugars. Its active site specifically binds to starch molecules, initiating digestion and highlighting enzyme-substrate specificity.
Example 2: Lactase
This enzyme breaks down lactose into glucose and galactose. Its structure enables it to specifically bind lactose, demonstrating enzyme specificity and the importance of enzymes in digesting dietary components.
Example 3: Catalase
Found in cells, it decomposes hydrogen peroxide into water and oxygen. Its quaternary structure allows rapid conversion, preventing cellular damage from reactive oxygen species.
Example 4: DNA Polymerase
Essential for DNA replication, it catalyzes nucleotide addition to a DNA strand. Its active site accommodates nucleotides, ensuring accurate DNA synthesis and showcasing the role of enzymes in genetic processes.
Example 5: Pepsin
An enzyme in the stomach that breaks down proteins into peptides. Its acidic optimum pH aligns with stomach acidity, illustrating the influence of environmental factors on enzyme function.
Practice Questions
Question 1:
Which of the following best describes the role of enzymes in biochemical reactions?
A) They increase activation energy
B) They alter the equilibrium constant
C) They decrease the activation energy
D) They change the overall energy of the reaction
Answer: C) They decrease the activation energy
Explanation: Enzymes lower the activation energy required for reactions, making them faster. They do not alter equilibrium or overall energy changes, only the energy needed to initiate the reaction.*
Question 2:
What is the effect of a non-competitive inhibitor on an enzyme?
A) Decreases Km, no change in Vmax
B) Increases Km, no change in Vmax
C) Decreases Vmax, no change in Km
D) Increases both Km and Vmax
Answer: C) Decreases Vmax, no change in Km
Explanation: Non-competitive inhibitors bind allosterically, reducing the number of active enzymes. This lowers Vmax without affecting substrate affinity, hence no change in Km.*
Question 3:
Which factor would most likely lead to enzyme denaturation?
A) Low substrate concentration
B) Optimal temperature
C) Excessive pH changes
D) Presence of cofactors
Answer: C) Excessive pH changes
Explanation: Extreme pH changes disrupt enzyme structure, particularly the active site, leading to denaturation. Optimal temperatures and cofactors generally enhance enzyme activity, not denature it.