Equilibrium plays a vital role in chemical and biological systems, representing a state where forward and reverse reactions occur at the same rate. On the MCAT, you’ll explore the principles of dynamic equilibrium, Le Châtelier’s principle, and equilibrium constants (K_eq). Understanding the relationship between equilibrium and thermodynamics, how changes in concentration or pressure shift reactions, and the application of equilibrium in biological processes is essential for mastering the chemistry and biochemistry sections of the exam.
Learning Objectives
In studying "Equilibrium" for the MCAT, you should learn to differentiate between static and dynamic equilibrium, focusing on how systems balance forces and maintain stability. Understand the concept of chemical equilibrium, including how the forward and reverse reaction rates become equal, and the role of the equilibrium constant K. Analyze how Le Châtelier’s principle predicts system responses to changes in concentration, pressure, temperature, and volume. Evaluate the importance of equilibrium in biological and chemical systems, such as enzyme kinetics and acid-base reactions. Additionally, develop problem-solving skills to interpret equilibrium graphs, calculate equilibrium concentrations, and apply equilibrium concepts in MCAT scenarios.
Differentiating Between Static and Dynamic Equilibrium
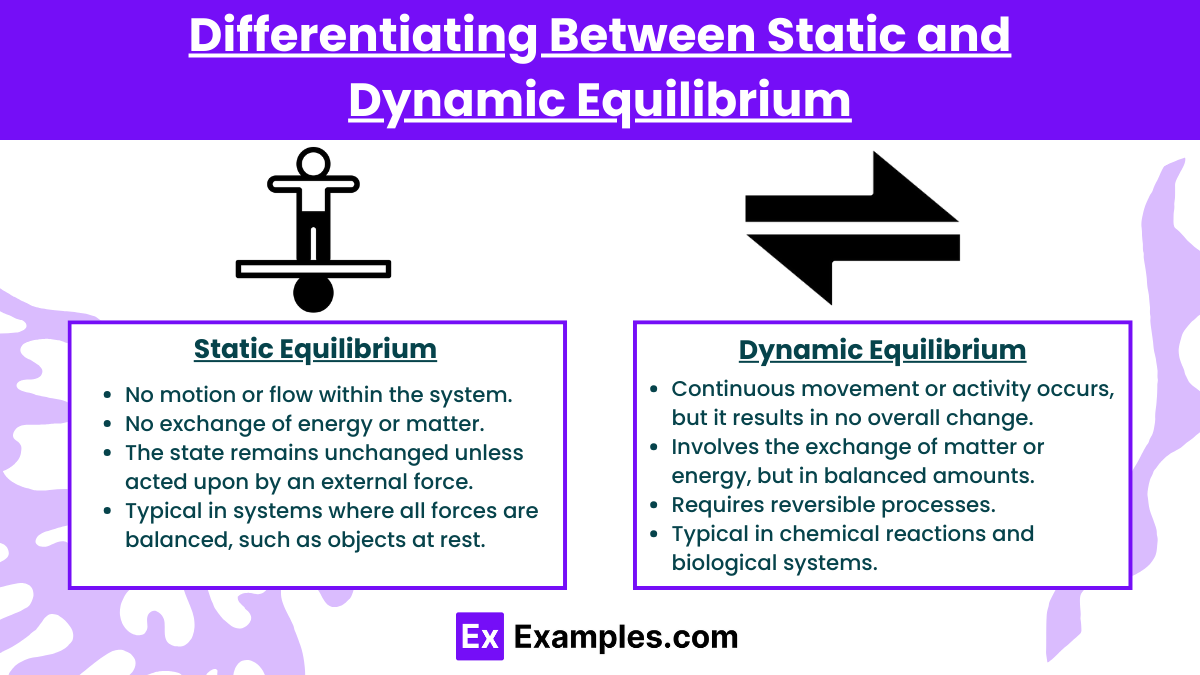
Equilibrium refers to a state in which all competing influences are balanced, resulting in no net change. However, equilibrium can be categorized into two main types: static equilibrium and dynamic equilibrium. Both represent balance, but they differ in how the components interact over time.
1. Static Equilibrium
A system is in static equilibrium when all forces or influences are balanced, and the system remains completely at rest with no movement or change over time.
Characteristics:
No motion or flow within the system.
No exchange of energy or matter.
The state remains unchanged unless acted upon by an external force.
Typical in systems where all forces are balanced, such as objects at rest.
Examples:
A book resting on a table: The gravitational force pulling the book downward is balanced by the normal force from the table.
A suspended object in balance: If a weight is hung by a string, and the tension in the string balances the gravitational force, the system remains static.
2. Dynamic Equilibrium
A system is in dynamic equilibrium when opposing processes occur simultaneously at equal rates, leading to no net change in the system's state over time.
Characteristics:
Continuous movement or activity occurs, but it results in no overall change.
Involves the exchange of matter or energy, but in balanced amounts.
Requires reversible processes.
Typical in chemical reactions and biological systems.
Examples:
Chemical Reactions: In a reversible reaction like N2+3H2 ⇌ 2NH3, the formation and decomposition of ammonia happen simultaneously at the same rate.
Water in a Closed Container: Water molecules evaporate from the liquid phase into vapor, while vapor molecules condense back into the liquid phase at the same rate.
Biological Systems: In the human body, dynamic equilibrium maintains homeostasis, such as keeping blood glucose levels stable by balancing insulin and glucagon release.
Understanding Chemical Equilibrium
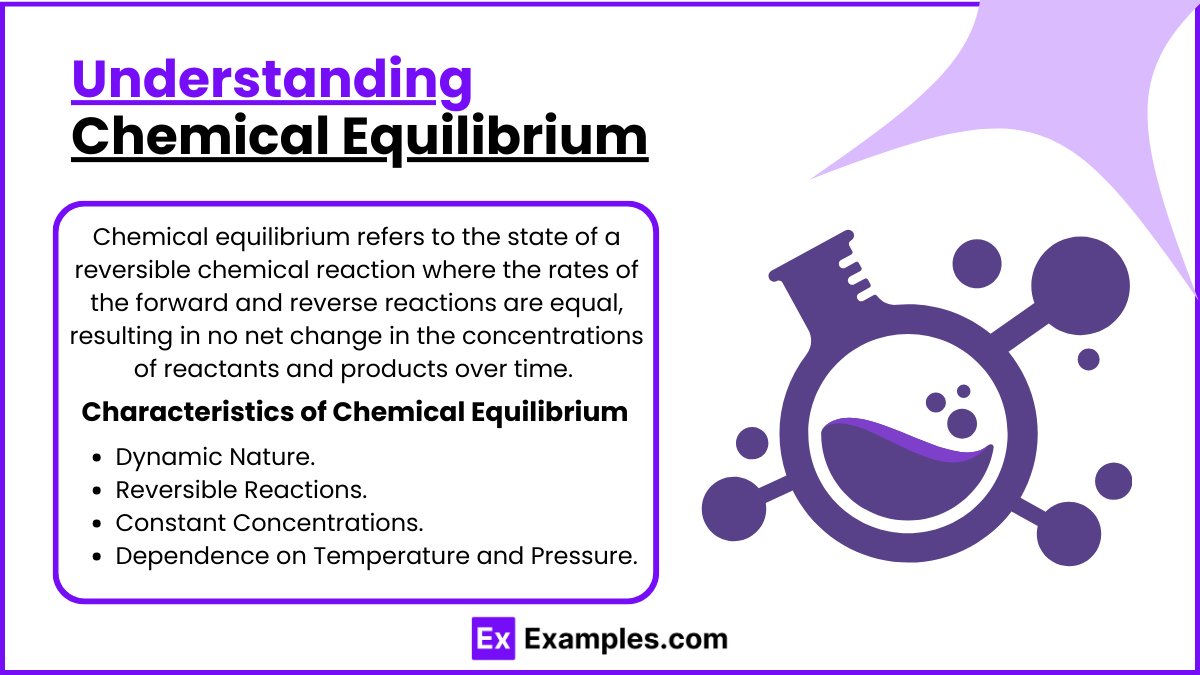
Chemical equilibrium refers to the state of a reversible chemical reaction where the rates of the forward and reverse reactions are equal, resulting in no net change in the concentrations of reactants and products over time. Even though the individual reactions continue, their effects cancel out, creating a stable, dynamic state.
Characteristics of Chemical Equilibrium
Dynamic Nature: Even though the system appears stable, both the forward and reverse reactions continue to occur at equal rates, maintaining constant concentrations of reactants and products.
Reversible Reactions: Chemical equilibrium only occurs in reversible reactions, which can proceed in both the forward (reactants to products) and reverse (products to reactants) directions.
Constant Concentrations: At equilibrium, the concentrations of reactants and products remain unchanged, although they are not necessarily equal.
Dependence on Temperature and Pressure: Changes in external conditions like temperature, pressure, and concentration can shift the equilibrium position, favoring either the forward or reverse reaction.
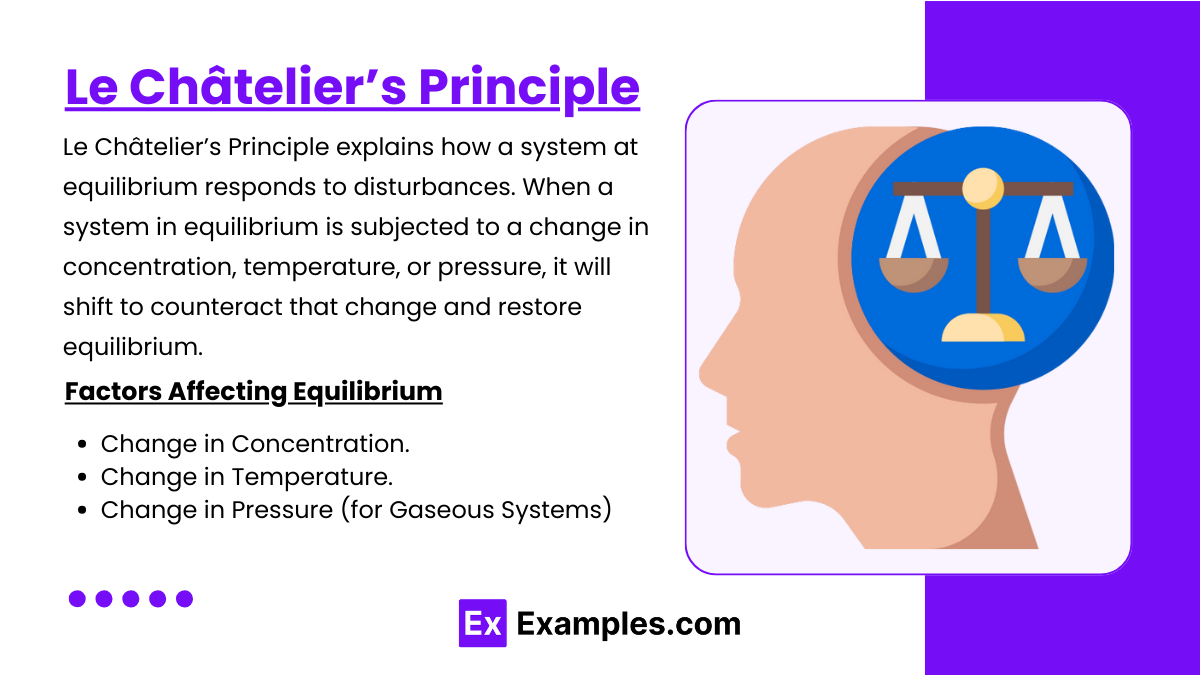
Le Châtelier’s Principle
Le Châtelier’s Principle explains how a system at equilibrium responds to disturbances. When a system in equilibrium is subjected to a change in concentration, temperature, or pressure, it will shift to counteract that change and restore equilibrium.
Change in Concentration:
Adding more reactants shifts the equilibrium toward the products.
Removing products shifts the equilibrium toward the products as well.
Change in Temperature:
Increasing temperature shifts the equilibrium in the direction of the endothermic reaction (absorbs heat).
Decreasing temperature shifts the equilibrium toward the exothermic reaction (releases heat).
Change in Pressure (for Gaseous Systems):
Increasing pressure shifts the equilibrium toward the side with fewer gas molecules.
Decreasing pressure shifts the equilibrium toward the side with more gas molecules.
Factors Affecting Equilibrium
Change in Concentration
Adding reactants: The system shifts toward the products to reduce the added reactant.
Removing reactants: The system shifts toward the reactants to compensate for the loss.
Adding products: The equilibrium shifts toward the reactants to reduce the excess product.
Removing products: The equilibrium shifts toward the products to replace the removed product.
Example: In the synthesis of ammonia:
N2(g)+3H2(g) ⇌ 2NH3(g)
Change in Temperature
Exothermic reactions (release heat):
Increasing temperature shifts the equilibrium toward the reactants.
Decreasing temperature shifts the equilibrium toward the products.
Endothermic reactions (absorb heat):
Increasing temperature shifts the equilibrium toward the products.
Decreasing temperature shifts the equilibrium toward the reactants.
Example: The decomposition of calcium carbonate is endothermic:
CaCO3(s) ⇌ CaO(s)+CO2(g)
Increasing temperature favors the decomposition of calcium carbonate into calcium oxide and carbon dioxide.
Change in Pressure (For Gaseous Reactions)
Increasing pressure shifts the equilibrium toward the side with fewer gas molecules.
Decreasing pressure shifts the equilibrium toward the side with more gas molecules.
Example: In the reaction:
N2(g)+3H2(g) ⇌ 2NH3(g)
Increasing pressure shifts the equilibrium toward ammonia production because there are fewer gashttps://www.examples.com/mcat/reflection-and-refraction molecules (2 moles) on the product side compared to the reactant side (4 moles).
Effect of Catalysts
Catalysts speed up the forward and reverse reactions equally, helping the system reach equilibrium faster.
However, catalysts do not change the position of equilibrium or the concentrations of reactants and products.
Importance of Equilibrium in Biological and Chemical Systems
Equilibrium plays a crucial role in both biological and chemical systems, as it governs the balance between reactants and products in reactions that are essential for life and industrial processes. Understanding equilibrium helps in optimizing conditions for reactions, maintaining homeostasis in organisms, and developing sustainable chemical processes.
Biological systems rely on equilibrium to maintain stability and ensure that biochemical processes function efficiently. Some critical areas where equilibrium plays a role include:
a. Homeostasis
Homeostasis refers to the body’s ability to maintain a stable internal environment despite external changes.
Example: Blood pH is regulated around 7.4. A shift in equilibrium between carbon dioxide, carbonic acid, and bicarbonate ions helps maintain this pH.
CO2+H2O ⇌ H2CO3 ⇌ H++HCO3−
b. Enzyme Activity
Enzymes facilitate biochemical reactions by lowering the activation energy. Equilibrium between substrates and products ensures efficient conversion and regulation of metabolic pathways.
Example: In glycolysis, the reversible reaction between glucose and glucose-6-phosphate ensures a steady supply of energy based on the cell's needs.
c. Oxygen Transport in Blood
The equilibrium between hemoglobin, oxygen, and carbon dioxide in the blood regulates oxygen delivery to tissues. Changes in pH and CO₂ levels shift the equilibrium, ensuring tissues receive enough oxygen under varying conditions.
Hb+4O2 ⇌ Hb(O2)4
d. Cellular Respiration
Cellular respiration involves multiple equilibrium steps in which glucose is gradually broken down to produce ATP. The equilibrium between reactants and products in the electron transport chain helps maintain a steady production of energy.
Examples
Example 1: Balanced Forces on a Hanging Object
Consider a picture frame hanging on a wall. The gravitational force pulling the frame downward is balanced by the tension in the supporting wire or string, resulting in a state of equilibrium. Since the net force acting on the frame is zero, it remains stationary, illustrating the concept of static equilibrium.
Example 2: A Book on a Table
When a book rests on a table, it is in a state of equilibrium. The weight of the book exerts a downward force due to gravity, while the table provides an equal and opposite normal force. This balance of forces ensures that the book does not move, demonstrating how equilibrium is achieved when opposing forces are equal.
Example 3: Chemical Equilibrium in Reactions
In a chemical reaction, equilibrium occurs when the rate of the forward reaction equals the rate of the reverse reaction. For example, in the synthesis of ammonia from nitrogen and hydrogen, when the concentrations of reactants and products remain constant over time, the system is said to be in chemical equilibrium. This principle is fundamental in understanding dynamic systems in chemistry.
Example 4: Equilibrium in a Beam
A horizontal beam supported at both ends experiences equilibrium when the downward forces acting on it (such as the weight of objects placed on it) are balanced by the upward forces exerted by the supports. If the beam is perfectly horizontal and not rotating, it is in a state of rotational equilibrium, where the sum of the torques about any point is zero.
Example 5: Market Equilibrium in Economics
In economics, market equilibrium occurs when the quantity of a good supplied equals the quantity demanded at a certain price level. For instance, if the supply of apples matches consumer demand at a price of $1 per apple, the market is in equilibrium. This balance ensures that there is neither a surplus nor a shortage, leading to a stable market condition.
Practice Questions
Question 1
Which of the following statements accurately describes static equilibrium?
A) An object in motion is in static equilibrium.
B) The net force acting on an object is zero.
C) The object is experiencing acceleration.
D) The object is changing shape.
Correct Answer: B) The net force acting on an object is zero.
Explanation: Static equilibrium refers to a state where an object is at rest and the net force acting on it is zero. This means that all the forces acting on the object are balanced, resulting in no movement. Option A is incorrect because static equilibrium specifically applies to objects at rest, not in motion. Option C is false, as an object in static equilibrium cannot be accelerating. Option D is also incorrect since the object remains unchanged in shape while in equilibrium.
Question 2
In a chemical reaction at equilibrium, which of the following statements is true?
A) The concentrations of reactants and products are equal.
B) The forward and reverse reactions occur at different rates.
C) No further changes occur in the concentrations of reactants and products.
D) The reaction stops completely.
Correct Answer: C) No further changes occur in the concentrations of reactants and products.
Explanation: At equilibrium, the rates of the forward and reverse reactions are equal, leading to constant concentrations of both reactants and products. However, this does not mean that the concentrations are equal, as they can vary. Option A is incorrect; concentrations do not need to be equal at equilibrium. Option B is misleading since the rates are equal, and option D is false because reactions continue to occur at equal rates even at equilibrium.
Question 3
What type of equilibrium is illustrated when a beam rests horizontally on two supports with weights distributed evenly along its length?
A) Dynamic equilibrium
B) Static equilibrium
C) Chemical equilibrium
D) Rotational equilibrium
Correct Answer: B) Static equilibrium.
Explanation: In this scenario, the beam is not moving and is supported evenly at both ends, demonstrating static equilibrium. All forces acting on the beam, including its weight and the normal forces from the supports, are balanced. This means there is no net force or motion, defining the state of static equilibrium. Option A refers to a state where an object is in motion but remains at constant velocity. Option C relates to chemical reactions, and option D is specific to rotational forces, which are also balanced in this case, but the primary state is static equilibrium.