A purchase flow chart, often referred to as a flow diagram, is a graphic representation of a system or a process detailing the sequencing of steps necessary to create an output. Typically, a flow chart uses a set of basic symbols so as to represent various functions. Also, it shows the sequence as well as how functions are interconnected using lines and arrows. You can use flow charts to document just about any kind of business system. In this article, we let you in on 10+ purchase flow chart examples and templates that you can us as a basis for creating your own. Keep on reading to learn more.
[bb_toc content=”][/bb_toc]
Purchase Flow Chart Examples & Templates
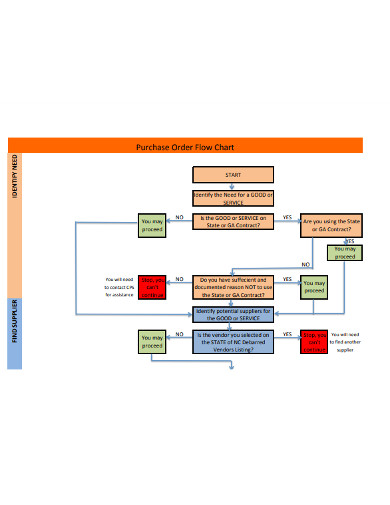
1. Purchase Order Flow Chart
This purchase order flow chart enables you to picture a process for your business. The processes are often specific to your own business but the good thing is that you can customize the templates so they fit your own business needs. This purchase flow chart example comes in different formats like PDF, Ms Word and Excel among others. They are also easily editable and no special program is required for you to make the changes.
With this purchase order flow chart example, creating your own flow diagram becomes very easy. You do not need to hire someone to do it for you as the template literally guides you on what to do.
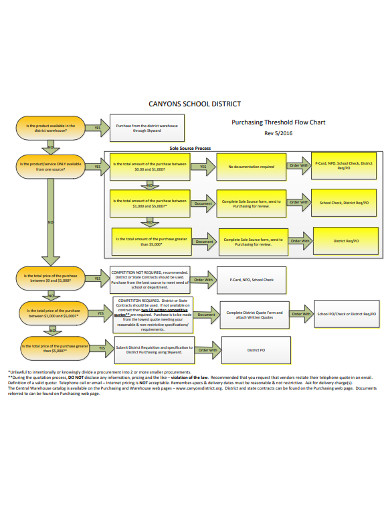
2. Purchasing Threshold Flow Chart
Need to create a purchasing threshold flowchart but not sure where to start? Do not worry. This example of a flow chart is all you need to quickly create a flowchart that you can use for business presentations. With the help of this template, you will make a professional-grade flowchart that you can share with your bosses or other stakeholders. After that, you can publish your flow chart as an image or a PDF. Also, it is easy to include the flowchart in a presentation or a report. Nothing makes creating a flowchart quite interesting like this template.
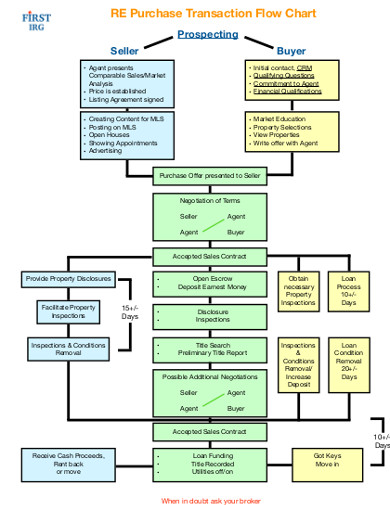
3. Purchase Transaction Flow Chart
If you are really stuck with your flow chart, you will find this filled purchase transaction flow chart a godsend. This flow diagram, which is available in an easily editable PDF file, makes a minced meat of creating a flow diagram. It is a ready-made template, leaving you with the work of only filling in the blank spaces. The templates are highly customizable, so you can modify them easily to suit your unique business circumstances. Next time you want to create a flow diagram, you have where to start. Download this template to help you create a professional-standard purchase flow diagram that you can use for business presentations to your bosses.
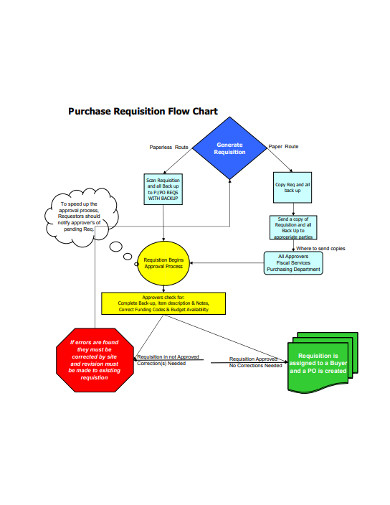
4. Purchase Requisition Flow Chart
Need to quickly prepare a purchase requisition flow chart to present to your bosses? There is no better place to start than this purchase requisition flow chart example. With the document, your work is made very easy. The flow diagram contains blank spaces where you only fill in your information and you are good to go. The template is also highly customizable, so you can add your logo and unique business features to make it look like it was created specifically for your business. Just download this template and quickly create a professional purchase requisition flow chart.
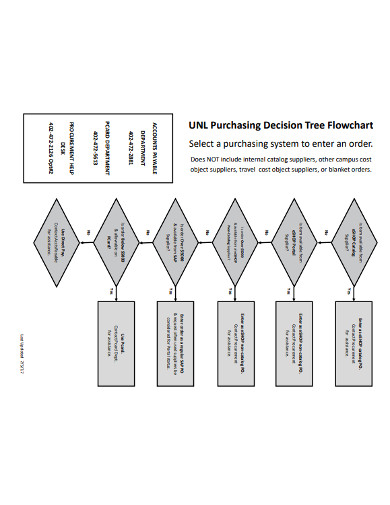
5. Purchasing Decision Tree Flowchart
Use this purchasing decision tree flow chart diagram to quickly generate a flowchart that you can make a presentation with. The flow chart is available in PDF format that you can easily edit and modify as you see fit. Also, customize this template by including your logo or letterhead to make it look like it was custom-made for your own business. The flow diagram is available on instant download. This means that you can download it anywhere you are and make a few edits and you have your flow diagram ready for presentation.
6. Purchase Order Request Procedure Flow Chart
With this purchase order request procedure flowchart example, your work of creating a flow diagram is done for you. What’s more, the flow diagram is available in instant download. This means that you do not need to be in your office to make this diagram. Just go to the internet, download it, make some edits, customize the flow chart and you are ready to make a presentation or send the flow chart to your bosses. Plus, the flowchart is easily editable, so no special programs are needed.
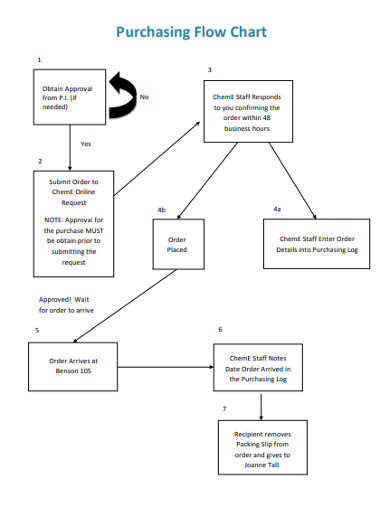
7. Purchasing Flow Chart in PDF
Use this purchasing flow chart example and template to quickly create a professional-looking diagram ready to send to your bosses. It is available on instant download, meaning you can access it anywhere without needing to be in your office. The file is easily editable and you will not need a special program to make changes to it. Also, it is customizable, allowing you to add your business logo and make the diagram look as if it was custom-made for you.
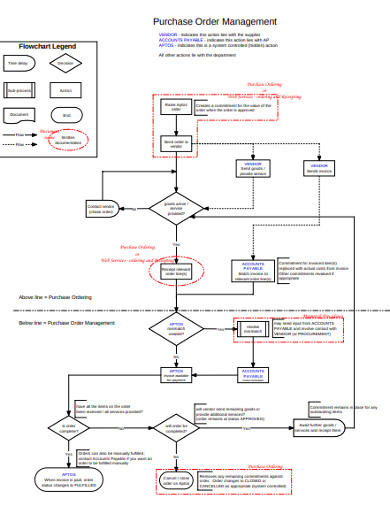
8. Purchase Order Management Flow Chart
is no better and faster way to make a purchase order flow chart than with this visually stunning purchase order management flow chart. Next time you are called to create this flow chart, you do not have to start from zero. Just use this template as a guide for making your own document in as little as a few minutes. The document is instantly available upon download, and you can easily make edits to it without using a special program.
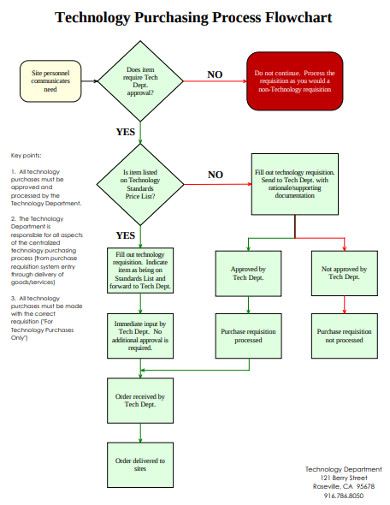
9. Technology Purchasing Process Flowchart
This is a purchasing process flowchart for people in the technology sector. If you are in that field, you will find that this document is tailor-made for you. It’s available on instant download and can be accessed from anywhere without you being at the office. Once you download it, just make some changes and add a logo and you have a document ready to send to your seniors. It really makes the job of creating a flow diagram easy.
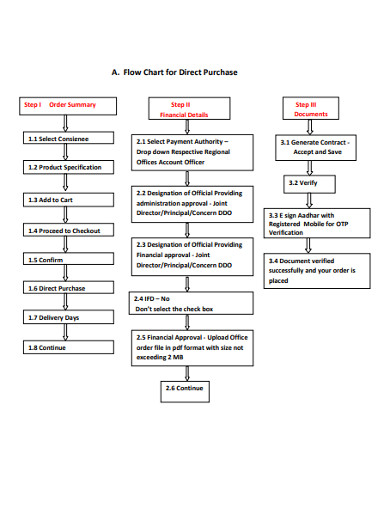
10. Flow Chart for Direct Purchase
Use this purchase flow chart example anytime you want to create a direct purchase flow chart. The flow diagram is available on PDF file that you can easily download and use to quickly create a professional-looking flow chart. Plus, it is easily editable, meaning no special program is required to edit it.
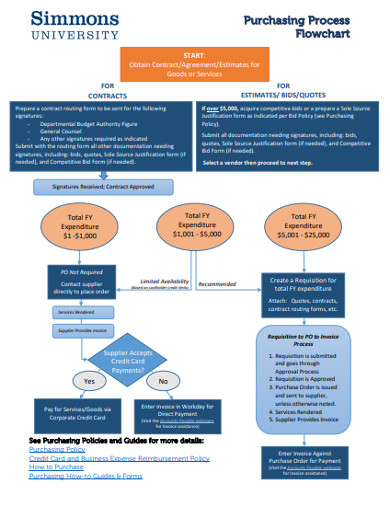
11. Purchasing Process Flowchart
This is a purchase order flow chart example that you can use as a basis for creating a professional-grade flow chart that you can then use in a presentation. Flow charts play a crucial role in helping people visualize a business process. When you want a better way of driving the point home, use this flow chart template for powerful illustration.