Kinetics

- Notes
Kinetics focuses on the rate of biochemical reactions and how enzymes influence these rates by lowering activation energy. Key concepts include the Michaelis-Menten equation, which explains how substrate concentration affects reaction velocity, and parameters like Km and Vmax, which indicate enzyme efficiency and substrate affinity. Different types of inhibition—competitive, non-competitive, and uncompetitive—alter enzyme activity in distinct ways. Kinetics also covers first-order and zero-order reactions, crucial in drug metabolism.
Learning Objectives
In studying “Kinetics” for the MCAT, you should learn to understand the principles of enzyme kinetics, including the Michaelis-Menten equation and factors affecting reaction rates. Analyze how parameters such as Km and Vmax describe enzyme-substrate affinity and catalytic efficiency. Evaluate different types of enzyme inhibition—competitive, non-competitive, and uncompetitive—using Lineweaver-Burk plots. Additionally, explore the role of first-order, zero-order, and allosteric kinetics in biological systems and clinical applications, such as drug metabolism. Apply these concepts to interpret kinetic data, predict reaction behavior, and solve enzyme-related problems in MCAT practice passages.
Introduction to Kinetics
Kinetics in biochemistry focuses on the rate at which biochemical reactions occur and how factors such as enzymes, substrate concentration, and inhibitors influence these rates. Enzyme kinetics is essential in understanding how enzymes catalyze reactions and the conditions that affect reaction velocity. These principles are critical for mastering MCAT concepts in biochemistry, molecular biology, and physiology.
Key Concepts in Enzyme Kinetics
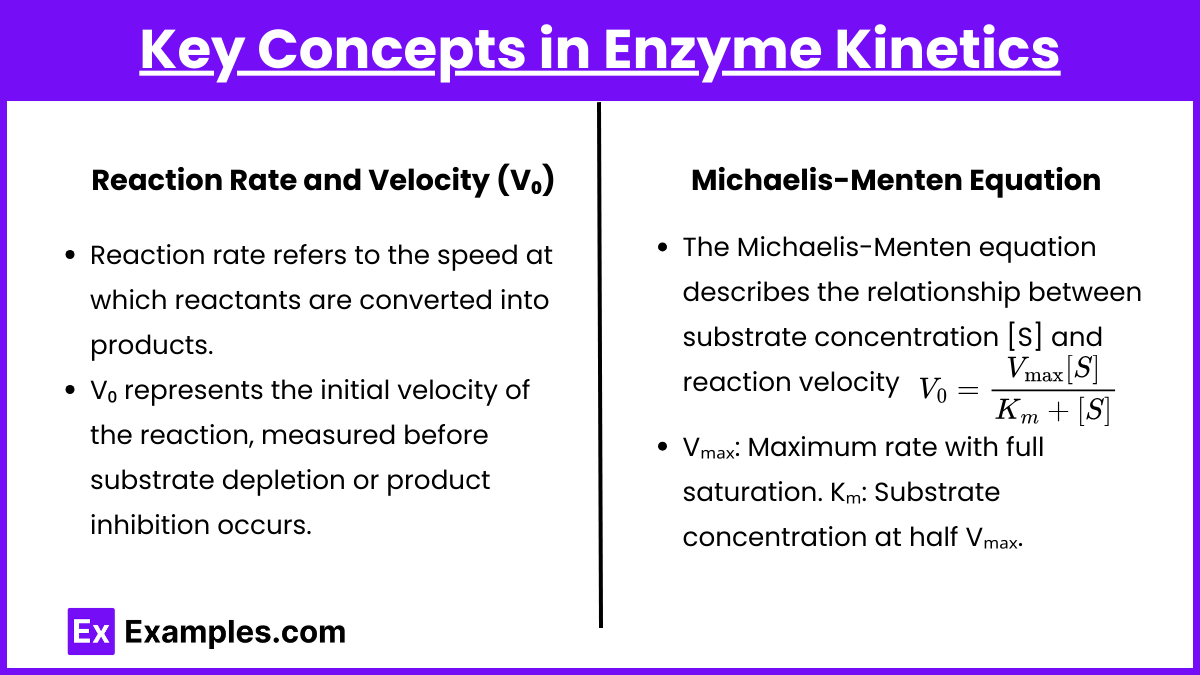
- Reaction Rate and Velocity (V₀):
- Reaction rate refers to the speed at which reactants are converted into products.
- V₀ represents the initial velocity of the reaction, measured before substrate depletion or product inhibition occurs.
- Michaelis-Menten Equation:
The Michaelis-Menten equation describes the relationship between substrate concentration [S] and reaction velocity V₀:.
- Vₘₐₓ : The maximum reaction rate when all enzyme active sites are saturated with substrate.
- Kₘ : The substrate concentration at which the reaction rate is half of Vmax. It reflects the enzyme’s affinity for the substrate—a lower Km indicates higher affinity.
Lineweaver-Burk Plot (Double-Reciprocal Plot)
The Lineweaver-Burk plot is a graphical method to determine Vmax and Km using the inverse of the Michaelis-Menten equation:
- The y-intercept represents
, and the x-intercept represents
.
- It helps differentiate between various types of enzyme inhibition.
Types of Enzyme Inhibition
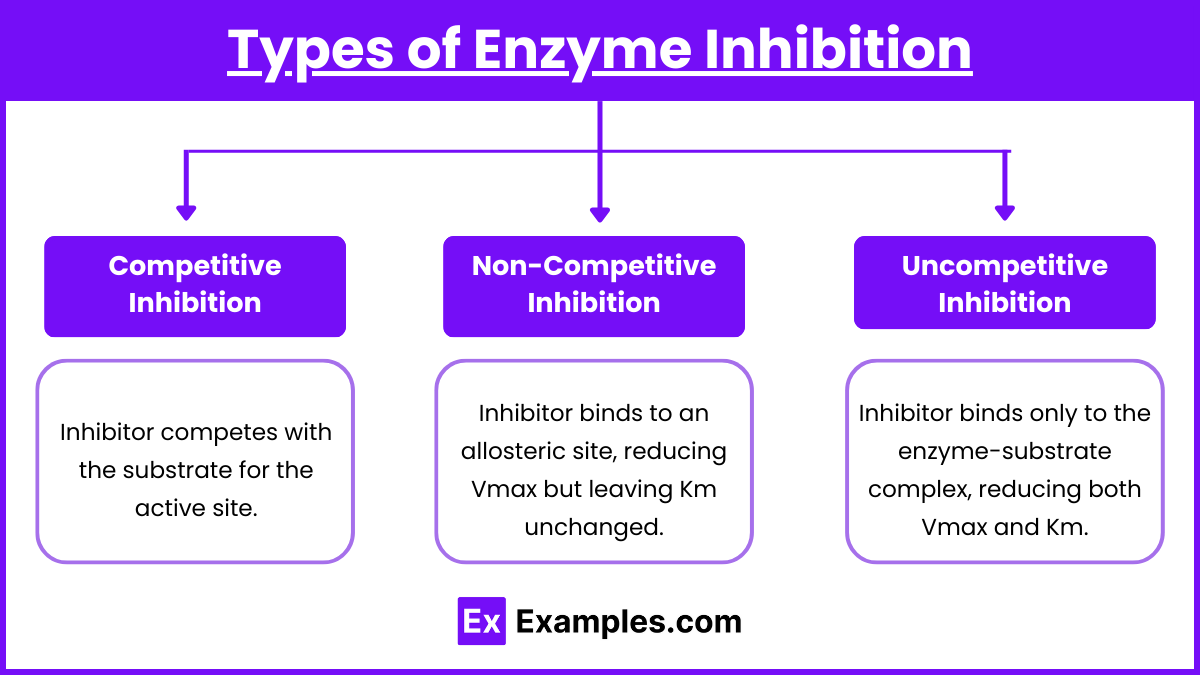
- Competitive Inhibition:
- Inhibitor competes with the substrate for the active site.
- Increases Km without affecting Vmax.
- Non-Competitive Inhibition:
- Inhibitor binds to an allosteric site, reducing Vmax but leaving Km unchanged.
- Uncompetitive Inhibition:
- Inhibitor binds only to the enzyme-substrate complex, reducing both Vmax and Km.
Factors Affecting Enzyme Kinetics
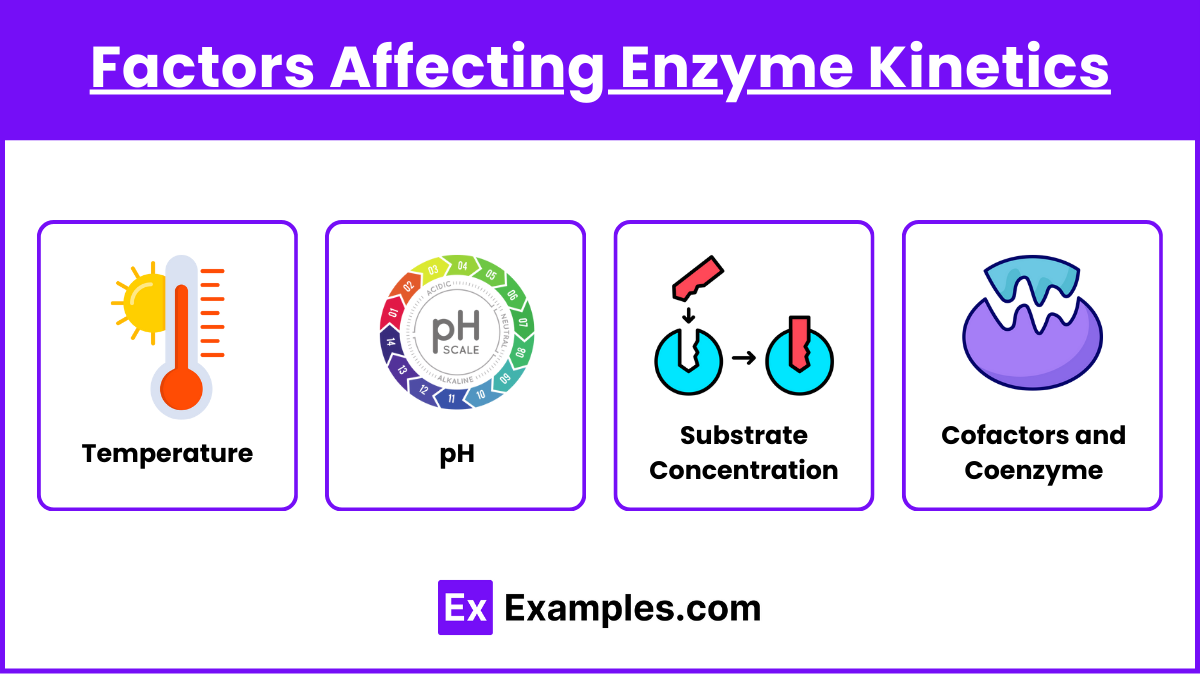
- Temperature:
- Increases kinetic energy and reaction rate, but extreme temperatures can denature enzymes.
- pH:
- Each enzyme functions optimally at a specific pH. Outside this range, enzyme activity decreases due to structural changes.
- Substrate Concentration:
- As substrate concentration increases, reaction rate increases until enzyme saturation is reached.
- Cofactors and Coenzymes:
- Some enzymes require metal ions (cofactors) or organic molecules (coenzymes) to function.
Examples
Example 1 : Michaelis-Menten Kinetics
This is the foundational model used to describe the rate of enzyme-catalyzed reactions. It illustrates how reaction velocity (V0) depends on substrate concentration ([S]) and provides insights into enzyme efficiency through parameters like Vmax (maximum velocity) and Km (substrate concentration at half Vmax). The MCAT frequently tests your ability to apply this model to interpret experimental data and compare enzyme behaviors.
Example 2 : First-Order Kinetics
In first-order kinetics, the reaction rate is directly proportional to the concentration of one reactant. For example, the breakdown of a drug in the bloodstream often follows first-order kinetics, where a constant fraction of the drug is metabolized per unit time. This concept is essential in pharmacokinetics, which explores drug absorption and elimination, a topic often encountered in MCAT passages.
Example 3 : Zero-Order Kinetics
In zero-order kinetics, the reaction rate remains constant and independent of reactant concentration. This occurs when enzymes or transport proteins are saturated, such as with high doses of alcohol, where the liver metabolizes ethanol at a constant rate regardless of concentration. MCAT questions may require you to distinguish between zero-order and first-order kinetics based on experimental results.
Example 4 : Lineweaver-Burk Plot
The Lineweaver-Burk plot is the double-reciprocal form of the Michaelis-Menten equation, providing a linear representation of enzyme kinetics. It is useful for determining Vmax and Km and identifying different types of enzyme inhibition. You may encounter MCAT questions requiring interpretation of Lineweaver-Burk graphs, such as distinguishing between competitive and non-competitive inhibition.
Example 5 : Allosteric Enzyme Kinetics
Allosteric enzymes do not follow Michaelis-Menten kinetics; instead, they display a sigmoidal (S-shaped) curve, indicating cooperative binding. These enzymes are regulated by activators or inhibitors that bind to allosteric sites, influencing enzyme activity. An example is hemoglobin, which shows cooperative binding of oxygen. Understanding these kinetic principles is crucial for the MCAT, particularly in questions related to enzyme regulation and metabolic control.
Practice Questions
Question 1
What does a low Km value indicate about an enzyme’s affinity for its substrate?
A) Low affinity for the substrate
B) High affinity for the substrate
C) The reaction is operating at maximum velocity
D) The enzyme activity is independent of substrate concentration
Correct Answer: B) High affinity for the substrate
Explanation: A low Km indicates that the enzyme reaches half of its maximum velocity (Vmax) at a low substrate concentration. This suggests that the enzyme binds efficiently to the substrate, meaning it has a high affinity. Option A is incorrect, as a high Km would indicate low affinity. Option C refers to Vmax, not Km. Option D is incorrect because enzyme activity does depend on substrate concentration, especially below saturation levels.
Question 2
Which type of kinetics is observed when the rate of reaction is independent of the concentration of the reactant?
A) First-order kinetics
B) Zero-order kinetics
C) Second-order kinetics
D) Michaelis-Menten kinetics
Correct Answer: B) Zero-order kinetics
Explanation: In zero-order kinetics, the reaction rate is constant and does not change with the concentration of the reactant. This typically occurs when the enzyme is saturated with substrate, and increasing the substrate concentration further does not affect the reaction rate. First-order kinetics (option A) involves a rate directly proportional to the reactant concentration. Second-order kinetics (option C) applies when the rate depends on the concentrations of two reactants. Michaelis-Menten kinetics (option D) describes how enzymes function over a range of substrate concentrations, including both first-order and zero-order behaviors.
Question 3
What type of inhibition is indicated by a Lineweaver-Burk plot where the lines intersect on the y-axis?
A) Competitive inhibition
B) Non-competitive inhibition
C) Uncompetitive inhibition
D) Mixed inhibition
Correct Answer: A) Competitive inhibition
Explanation: In competitive inhibition, the inhibitor competes with the substrate for binding to the active site, increasing the apparent Km without affecting Vmax. On a Lineweaver-Burk plot, this inhibition is shown by intersecting lines on the y-axis because only the slope (related to Km) changes, while the y-intercept (related to Vmax) remains constant. In non-competitive inhibition (option B), the Vmax decreases, but Km remains unaffected. Uncompetitive inhibition (option C) shows parallel lines. Mixed inhibition (option D) produces lines that intersect off the axes.
Kinetics focuses on the rate of biochemical reactions and how enzymes influence these rates by lowering activation energy. Key concepts include the Michaelis-Menten equation, which explains how substrate concentration affects reaction velocity, and parameters like Km and Vmax, which indicate enzyme efficiency and substrate affinity. Different types of inhibition—competitive, non-competitive, and uncompetitive—alter enzyme activity in distinct ways. Kinetics also covers first-order and zero-order reactions, crucial in drug metabolism.
Learning Objectives
In studying “Kinetics” for the MCAT, you should learn to understand the principles of enzyme kinetics, including the Michaelis-Menten equation and factors affecting reaction rates. Analyze how parameters such as Km and Vmax describe enzyme-substrate affinity and catalytic efficiency. Evaluate different types of enzyme inhibition—competitive, non-competitive, and uncompetitive—using Lineweaver-Burk plots. Additionally, explore the role of first-order, zero-order, and allosteric kinetics in biological systems and clinical applications, such as drug metabolism. Apply these concepts to interpret kinetic data, predict reaction behavior, and solve enzyme-related problems in MCAT practice passages.
Introduction to Kinetics
Kinetics in biochemistry focuses on the rate at which biochemical reactions occur and how factors such as enzymes, substrate concentration, and inhibitors influence these rates. Enzyme kinetics is essential in understanding how enzymes catalyze reactions and the conditions that affect reaction velocity. These principles are critical for mastering MCAT concepts in biochemistry, molecular biology, and physiology.
Key Concepts in Enzyme Kinetics

Reaction Rate and Velocity (V₀):
Reaction rate refers to the speed at which reactants are converted into products.
V₀ represents the initial velocity of the reaction, measured before substrate depletion or product inhibition occurs.
Michaelis-Menten Equation:
The Michaelis-Menten equation describes the relationship between substrate concentration [S] and reaction velocity V₀: V0=Km+[S]Vmax[S]$V_0 = \frac{V_{\text{max}}[S]}{K_m + [S]}$.Vₘₐₓ : The maximum reaction rate when all enzyme active sites are saturated with substrate.
Kₘ : The substrate concentration at which the reaction rate is half of Vmax. It reflects the enzyme’s affinity for the substrate—a lower Km indicates higher affinity.
Lineweaver-Burk Plot (Double-Reciprocal Plot)
The Lineweaver-Burk plot is a graphical method to determine Vmax and Km using the inverse of the Michaelis-Menten equation:
V01=Vmax[S]Km+Vmax1$\frac{1}{V_0} = \frac{K_m}{V_{\text{max}}[S]} + \frac{1}{V_{\text{max}}} $
The y-intercept represents 1/Vmax$1/V_{\text{max}}$, and the x-intercept represents −1/Km$-1/K_m$.
It helps differentiate between various types of enzyme inhibition.
Types of Enzyme Inhibition

Competitive Inhibition:
Inhibitor competes with the substrate for the active site.
Increases Km without affecting Vmax.
Non-Competitive Inhibition:
Inhibitor binds to an allosteric site, reducing Vmax but leaving Km unchanged.
Uncompetitive Inhibition:
Inhibitor binds only to the enzyme-substrate complex, reducing both Vmax and Km.
Factors Affecting Enzyme Kinetics

Temperature:
Increases kinetic energy and reaction rate, but extreme temperatures can denature enzymes.
pH:
Each enzyme functions optimally at a specific pH. Outside this range, enzyme activity decreases due to structural changes.
Substrate Concentration:
As substrate concentration increases, reaction rate increases until enzyme saturation is reached.
Cofactors and Coenzymes:
Some enzymes require metal ions (cofactors) or organic molecules (coenzymes) to function.
Examples
Example 1 : Michaelis-Menten Kinetics
This is the foundational model used to describe the rate of enzyme-catalyzed reactions. It illustrates how reaction velocity (V0) depends on substrate concentration ([S]) and provides insights into enzyme efficiency through parameters like Vmax (maximum velocity) and Km (substrate concentration at half Vmax). The MCAT frequently tests your ability to apply this model to interpret experimental data and compare enzyme behaviors.
Example 2 : First-Order Kinetics
In first-order kinetics, the reaction rate is directly proportional to the concentration of one reactant. For example, the breakdown of a drug in the bloodstream often follows first-order kinetics, where a constant fraction of the drug is metabolized per unit time. This concept is essential in pharmacokinetics, which explores drug absorption and elimination, a topic often encountered in MCAT passages.
Example 3 : Zero-Order Kinetics
In zero-order kinetics, the reaction rate remains constant and independent of reactant concentration. This occurs when enzymes or transport proteins are saturated, such as with high doses of alcohol, where the liver metabolizes ethanol at a constant rate regardless of concentration. MCAT questions may require you to distinguish between zero-order and first-order kinetics based on experimental results.
Example 4 : Lineweaver-Burk Plot
The Lineweaver-Burk plot is the double-reciprocal form of the Michaelis-Menten equation, providing a linear representation of enzyme kinetics. It is useful for determining Vmax and Km and identifying different types of enzyme inhibition. You may encounter MCAT questions requiring interpretation of Lineweaver-Burk graphs, such as distinguishing between competitive and non-competitive inhibition.
Example 5 : Allosteric Enzyme Kinetics
Allosteric enzymes do not follow Michaelis-Menten kinetics; instead, they display a sigmoidal (S-shaped) curve, indicating cooperative binding. These enzymes are regulated by activators or inhibitors that bind to allosteric sites, influencing enzyme activity. An example is hemoglobin, which shows cooperative binding of oxygen. Understanding these kinetic principles is crucial for the MCAT, particularly in questions related to enzyme regulation and metabolic control.
Practice Questions
Question 1
What does a low Km value indicate about an enzyme's affinity for its substrate?
A) Low affinity for the substrate
B) High affinity for the substrate
C) The reaction is operating at maximum velocity
D) The enzyme activity is independent of substrate concentration
Correct Answer: B) High affinity for the substrate
Explanation: A low Km indicates that the enzyme reaches half of its maximum velocity (Vmax) at a low substrate concentration. This suggests that the enzyme binds efficiently to the substrate, meaning it has a high affinity. Option A is incorrect, as a high Km would indicate low affinity. Option C refers to Vmax, not Km. Option D is incorrect because enzyme activity does depend on substrate concentration, especially below saturation levels.
Question 2
Which type of kinetics is observed when the rate of reaction is independent of the concentration of the reactant?
A) First-order kinetics
B) Zero-order kinetics
C) Second-order kinetics
D) Michaelis-Menten kinetics
Correct Answer: B) Zero-order kinetics
Explanation: In zero-order kinetics, the reaction rate is constant and does not change with the concentration of the reactant. This typically occurs when the enzyme is saturated with substrate, and increasing the substrate concentration further does not affect the reaction rate. First-order kinetics (option A) involves a rate directly proportional to the reactant concentration. Second-order kinetics (option C) applies when the rate depends on the concentrations of two reactants. Michaelis-Menten kinetics (option D) describes how enzymes function over a range of substrate concentrations, including both first-order and zero-order behaviors.
Question 3
What type of inhibition is indicated by a Lineweaver-Burk plot where the lines intersect on the y-axis?
A) Competitive inhibition
B) Non-competitive inhibition
C) Uncompetitive inhibition
D) Mixed inhibition
Correct Answer: A) Competitive inhibition
Explanation: In competitive inhibition, the inhibitor competes with the substrate for binding to the active site, increasing the apparent Km without affecting Vmax. On a Lineweaver-Burk plot, this inhibition is shown by intersecting lines on the y-axis because only the slope (related to Km) changes, while the y-intercept (related to Vmax) remains constant. In non-competitive inhibition (option B), the Vmax decreases, but Km remains unaffected. Uncompetitive inhibition (option C) shows parallel lines. Mixed inhibition (option D) produces lines that intersect off the axes.