Nucleic acids, lipids, and carbohydrates

- Notes
Nucleic acids, lipids, and carbohydrates are essential biological macromolecules that play key roles in the structure and function of cells. For the MCAT, understanding the structure, function, and interactions of these molecules is crucial for analyzing cellular processes, metabolism, and genetic information.
Learning Objectives
In studying nucleic acids, lipids, and carbohydrates for the MCAT, you should be able to identify the basic structures of these macromolecules, recognize their functions, and understand how they contribute to metabolism, energy storage, and information transmission in cells. You should also understand how these molecules are synthesized, broken down, and how they interact with other biomolecules.
What are Nucleic Acids?
Nucleic acids, such as DNA and RNA, are the molecules responsible for storing and transmitting genetic information. They are composed of nucleotide monomers, each consisting of a phosphate group, a sugar (deoxyribose in DNA or ribose in RNA), and a nitrogenous base (adenine, thymine/uracil, cytosine, or guanine). DNA serves as the genetic blueprint for life, while RNA plays a role in protein synthesis and other cellular processes.
Key Concepts:
- DNA Structure: Double-stranded helix with complementary base pairing (A-T, C-G).
- RNA Structure: Typically single-stranded, with uracil replacing thymine (A-U, C-G).
- Functions: DNA stores genetic information; RNA assists in protein synthesis and gene regulation.
What are Lipids?
Lipids are a diverse group of hydrophobic molecules that play roles in energy storage, membrane structure, and signaling. They include fats, oils, phospholipids, and steroids. Unlike nucleic acids and carbohydrates, lipids are not polymers but are composed of different types of hydrophobic and amphipathic molecules.
Key Concepts:
- Fatty Acids: Long hydrocarbon chains with a carboxyl group at one end. Can be saturated (no double bonds) or unsaturated (one or more double bonds).
- Phospholipids: Major components of cell membranes, consisting of two fatty acids, a phosphate group, and a glycerol backbone.
- Steroids: Lipids characterized by a four-ring structure, such as cholesterol and steroid hormones.
- Functions: Energy storage, insulation, cell membrane structure, and signaling.
What are Carbohydrates?
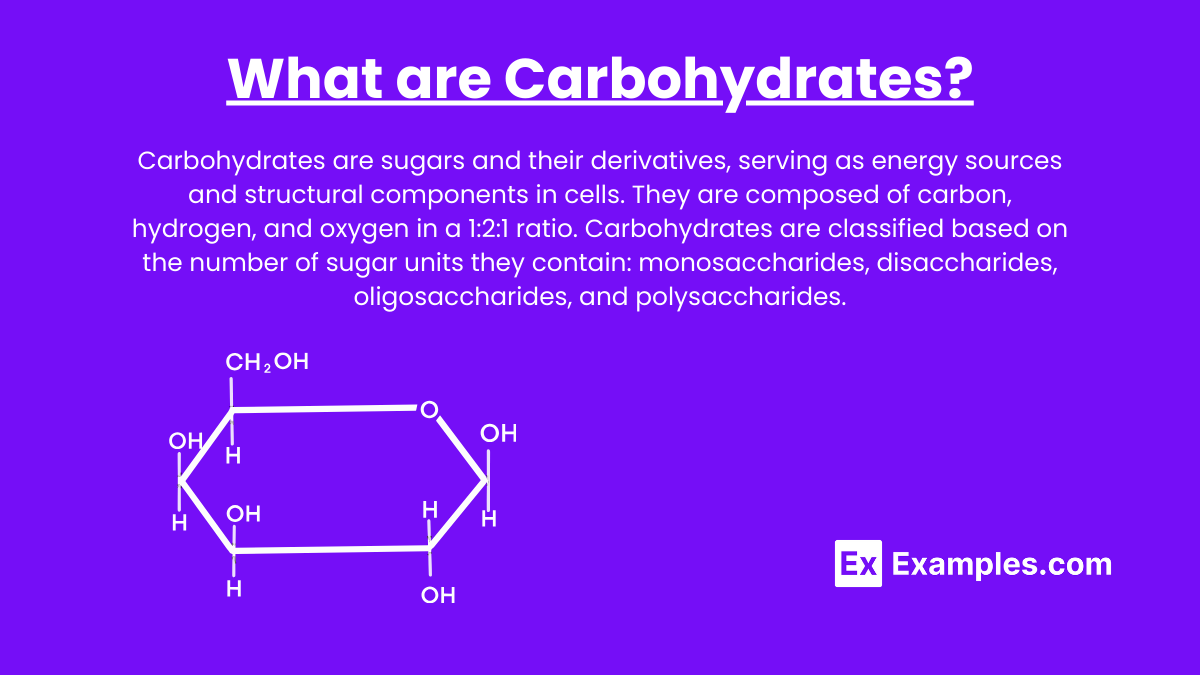
Carbohydrates are sugars and their derivatives, serving as energy sources and structural components in cells. They are composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. Carbohydrates are classified based on the number of sugar units they contain: monosaccharides, disaccharides, oligosaccharides, and polysaccharides.
Key Concepts:

- Monosaccharides: Simple sugars like glucose, fructose, and galactose.
- Disaccharides: Two monosaccharides linked together, such as sucrose (glucose + fructose) and lactose (glucose + galactose).
- Polysaccharides: Long chains of monosaccharides, including glycogen (energy storage in animals) and starch (energy storage in plants).
- Functions: Energy storage, providing structure (cellulose in plants), and cellular recognition.
Examples
Example 1: DNA Structure
DNA is composed of two strands of nucleotides, with each nucleotide containing a deoxyribose sugar, a phosphate group, and a nitrogenous base (A, T, C, or G). The strands are held together by hydrogen bonds between complementary bases (A-T and C-G), forming a double helix. DNA’s structure is crucial for its function in storing genetic information.
Example 2: Fatty Acid Structure
Fatty acids consist of a hydrocarbon chain and a carboxyl group. Saturated fatty acids have no double bonds and are solid at room temperature (e.g., butter). Unsaturated fatty acids, like oleic acid, have one or more double bonds, which create kinks in the chain and result in a liquid state at room temperature (e.g., olive oil).
Example 3: Glucose (Monosaccharide)
Glucose is a six-carbon monosaccharide with the chemical formula C₆H₁₂O₆. It is the primary source of energy for cells and is a key product of photosynthesis. Glucose is also the building block for polysaccharides like glycogen and starch.
Example 4: Phospholipid Bilayer
Phospholipids, which make up cell membranes, consist of a hydrophilic phosphate head and two hydrophobic fatty acid tails. In aqueous environments, they spontaneously form bilayers, with the hydrophobic tails facing inward and the hydrophilic heads facing outward, creating a selective barrier for cells.
Example 5: Cholesterol (Steroid Lipid)
Cholesterol is a steroid lipid with a four-ring structure that plays a vital role in maintaining the fluidity of cell membranes. It is also a precursor for the synthesis of steroid hormones like testosterone and estrogen, as well as bile acids that aid in digestion.
Practical Questions
Question 1:
Which of the following molecules serves as the main structural component of cell membranes?
A) DNA
B) Phospholipids
C) Glycogen
D) Cholesterol
Answer: B) Phospholipids
Explanation: Phospholipids form the lipid bilayer of cell membranes, with hydrophilic heads facing outward and hydrophobic tails facing inward, creating a selectively permeable barrier.
Question 2:
Which of the following pairs correctly matches the type of carbohydrate with an example?
A) Monosaccharide – Lactose
B) Disaccharide – Glucose
C) Monosaccharide – Fructose
D) Polysaccharide – Sucrose
Answer: C) Monosaccharide – Fructose
Explanation: Fructose is a monosaccharide. Lactose is a disaccharide, glucose is also a monosaccharide, and sucrose is a disaccharide.
Question 3:
Which of the following nucleotides is found only in RNA?
A) Adenine
B) Thymine
C) Cytosine
D) Uracil
Answer: D) Uracil
Explanation: Uracil is found only in RNA, where it replaces thymine. The other nitrogenous bases (adenine, cytosine, and guanine) are found in both DNA and RNA.