The periodic table is essential to understanding chemical elements and their properties, serving as a foundation for topics in chemistry and biochemistry. On the MCAT, you’ll explore trends such as electronegativity, ionization energy, and atomic radii, along with classifications like metals, nonmetals, and metalloids. Mastering periodic trends, electron configurations, and reactivity is crucial, as these concepts play a significant role in understanding chemical bonding, reactions, and molecular interactions throughout the exam.
Learning Objectives
In studying "Periodic Table" for the MCAT, you should learn to understand the structure and organization of elements based on atomic number, electron configurations, and recurring chemical properties. Analyze trends such as atomic radius, ionization energy, electron affinity, and electronegativity across periods and groups. Recognize the significance of element groups like alkali metals, alkaline earth metals, halogens, and noble gases. Evaluate the relationship between an element’s position on the table and its chemical reactivity and bonding behavior. Additionally, apply periodic trends to predict physical and chemical properties, reaction outcomes, and molecular structure, which are essential for MCAT problem-solving.
Understanding the Structure and Organization of the Periodic Table
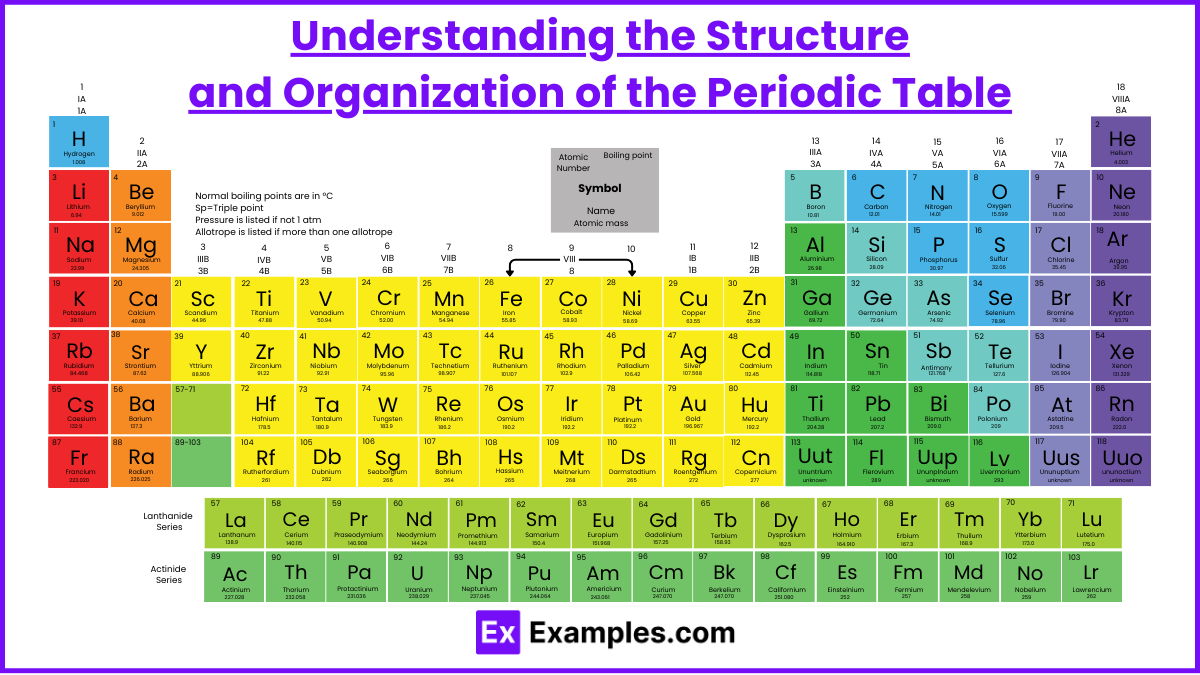
The periodic table organizes all known elements based on their atomic structure and chemical properties. This arrangement makes it easy to predict how elements will behave in chemical reactions.
1. Basic Layout
Rows (Periods):
The horizontal rows are called periods.
As you move across a period from left to right, the number of protons and electrons increases by one for each element.
Columns (Groups or Families):
The vertical columns are called groups or families.
Elements in the same group have similar chemical properties because they have the same number of valence electrons (outermost electrons).
2. Organization by Atomic Number
Atomic number = number of protons in an atom’s nucleus.
Elements are arranged in increasing atomic number from left to right and top to bottom.
3. Metals, Non-Metals, and Metalloids
Metals:
Found on the left and middle of the periodic table.
Good conductors of heat and electricity, malleable, and tend to lose electrons (forming positive ions).
Non-Metals:
Found on the right side of the table (except hydrogen).
Poor conductors, brittle, and tend to gain electrons (forming negative ions).
Metalloids:
Found along the zigzag line (stair-step) separating metals and non-metals.
Exhibit properties of both metals and non-metals (like silicon).
4. Groups and Their Properties
Group 1 (Alkali Metals): 1 valence electron, highly reactive (e.g., sodium).
Group 2 (Alkaline Earth Metals): 2 valence electrons, reactive but less than alkali metals (e.g., calcium).
Group 17 (Halogens): 7 valence electrons, highly reactive non-metals (e.g., chlorine).
Group 18 (Noble Gases): Full outer electron shells, very stable and unreactive (e.g., helium, neon).
5. Trends in the Periodic Table
Atomic Radius: Increases down a group, decreases across a period.
Ionization Energy: Decreases down a group, increases across a period (how hard it is to remove an electron).
Electronegativity: Decreases down a group, increases across a period (how strongly an atom attracts electrons).
Analyzing Periodic Trends Across Periods and Groups
The periodic table shows repeating patterns (or trends) in the physical and chemical properties of elements. These periodic trends arise from changes in atomic structure as you move across periods (rows) or down groups (columns). Understanding these trends helps predict how elements behave in chemical reactions.
1. Atomic Radius (Size of the Atom)
Across a Period (Left to Right):
Atomic radius decreases because more protons in the nucleus attract the electrons more strongly, pulling them closer to the nucleus.
Example: Sodium (Na) is larger than chlorine (Cl) within the same period.
Down a Group (Top to Bottom):
Atomic radius increases because new energy levels (electron shells) are added, making the atom larger.
Example: Lithium (Li) is smaller than cesium (Cs) in Group 1.
2. Ionization Energy (Energy to Remove an Electron)
Across a Period:
Ionization energy increases because the atoms become smaller, and the electrons are held more tightly by the nucleus. It takes more energy to remove an electron.
Example: Fluorine (F) has a higher ionization energy than lithium (Li).
Down a Group:
Ionization energy decreases because the outer electrons are farther from the nucleus and easier to remove due to weaker attraction.
Example: Potassium (K) has a lower ionization energy than lithium (Li).
3. Electronegativity (Tendency to Attract Electrons)
Across a Period:
Electronegativity increases as atoms become smaller and their nuclei attract electrons more strongly.
Example: Oxygen (O) is more electronegative than carbon (C).
Down a Group:
Electronegativity decreases because atoms become larger, and the attraction for electrons weakens.
Example: Fluorine (F) is more electronegative than iodine (I).
4. Metallic and Non-Metallic Character
Across a Period:
Metallic character decreases (elements become less like metals) as atoms hold on to their electrons more tightly.
Example: Sodium (Na) is metallic, but chlorine (Cl) is non-metallic.
Down a Group:
Metallic character increases as atoms lose electrons more easily.
Example: Cesium (Cs) is more metallic than lithium (Li).
5.. Electron Affinity (Energy Released When an Atom Gains an Electron)
Across a Period:
Electron affinity increases, meaning atoms more readily accept electrons as they approach a full valence shell.
Example: Chlorine (Cl) releases more energy when gaining an electron than sodium (Na).
Down a Group:
Electron affinity decreases because larger atoms have weaker attractions for new electrons.
Example: Fluorine (F) has higher electron affinity than bromine (Br).
Significance of Element Groups
The groups (or columns) of the periodic table play a crucial role in understanding the chemical behavior and properties of elements. Elements in the same group share similar properties because they have the same number of valence electrons, which determine how they participate in chemical reactions.
Key Groups and Their Properties
Group 1: Alkali Metals
Valence Electrons: 1
Properties:
Very reactive, especially with water, forming hydroxides and releasing hydrogen gas.
Soft metals that can be cut with a knife.
Reactivity increases down the group (e.g., lithium < sodium < potassium).
Examples: Lithium (Li), Sodium (Na), Potassium (K)
Significance: Used in batteries (e.g., lithium-ion batteries) and biological processes (e.g., sodium-potassium pumps in cells).
Group 17: Halogens
Valence Electrons: 7
Properties:
Highly reactive non-metals.
Reactivity decreases down the group (e.g., fluorine > chlorine > bromine).
Form salts when combined with metals (e.g., NaCl).
Examples: Fluorine (F), Chlorine (Cl), Iodine (I)
Significance: Used in water purification (chlorine), disinfectants, and pharmaceuticals.
Group 18: Noble Gases
Valence Electrons: 8 (except Helium, with 2)
Properties:
Extremely stable and inert (non-reactive) due to their full valence shells.
Low boiling points, existing as gases at room temperature.
Examples: Helium (He), Neon (Ne), Argon (Ar)
Significance: Used in lighting (neon signs), cooling systems (liquid helium), and inert environments (argon in welding).
Transition Metals (Groups 3-12)
Valence Electrons: Vary, typically involve d-orbitals.
Properties:
Exhibit multiple oxidation states.
Good conductors of heat and electricity.
Often form colorful compounds.
Examples: Iron (Fe), Copper (Cu), Gold (Au)
Significance: Used in construction (iron and steel), electrical wiring (copper), and jewelry (gold).
Why Groups Matter
Chemical Reactivity:
Elements within a group behave similarly in chemical reactions because they have the same number of valence electrons.
Periodic Trends:
Groups help predict trends, such as reactivity, ionization energy, and atomic radius, making it easier to understand and anticipate an element's behavior.
Industrial and Biological Applications:
Understanding group properties allows for the proper use of elements in technology (e.g., alkali metals in batteries) and health (e.g., calcium in bones).
Predicting New Elements:
Chemists can predict the properties of unknown or newly discovered elements based on their group position.
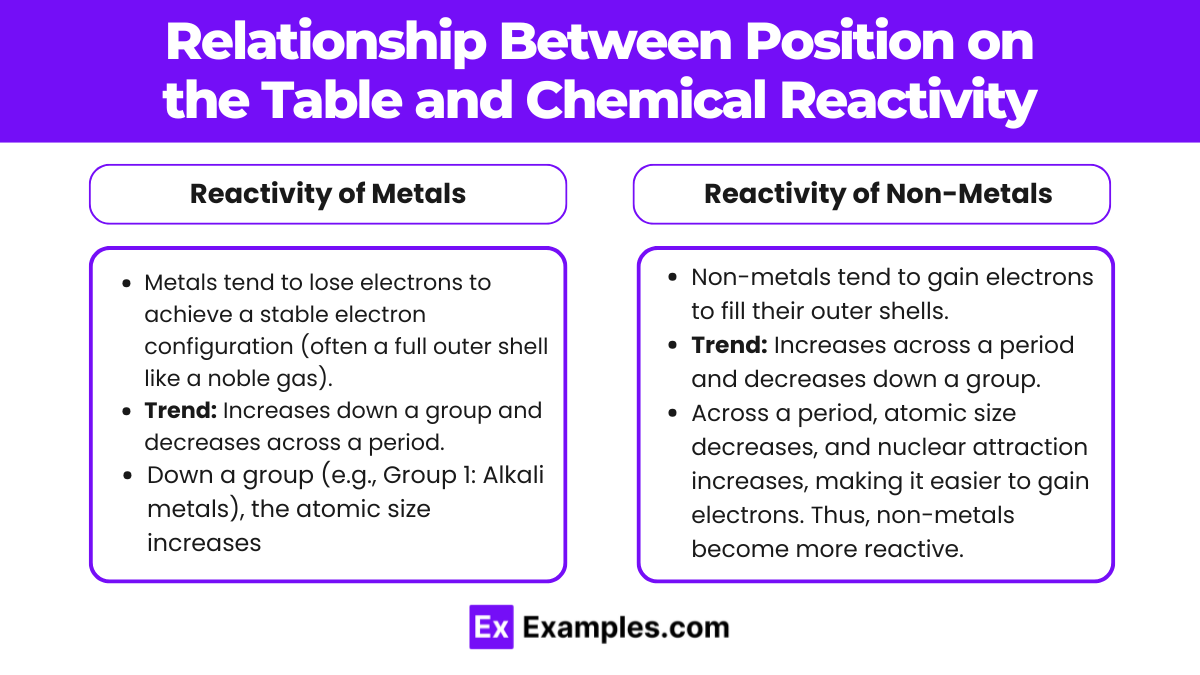
Relationship Between Position on the Table and Chemical Reactivity
The position of an element on the periodic table plays a crucial role in determining its chemical reactivity—how easily an element participates in chemical reactions. Reactivity trends differ for metals and non-metals based on their electron configurations and how easily they gain or lose electrons.
Reactivity of Metals
Metals tend to lose electrons to achieve a stable electron configuration (often a full outer shell like a noble gas).
Trend: Increases down a group and decreases across a period.
Down a group (e.g., Group 1: Alkali metals), the atomic size increases, and the outer electrons are farther from the nucleus. This makes it easier to lose electrons, increasing reactivity.
Example: Potassium (K) is more reactive than lithium (Li).
Across a period from left to right, the atomic size decreases, and nuclear attraction increases. As a result, it becomes harder to lose electrons, decreasing reactivity.
Example: Sodium (Na) is more reactive than magnesium (Mg) in the same period.
Most Reactive Metals:
Alkali Metals (Group 1) are the most reactive metals because they have only one valence electron to lose.
Example: Cesium (Cs) and francium (Fr) are among the most reactive metals.
Reactivity of Non-Metals
Non-metals tend to gain electrons to fill their outer shells.
Trend: Increases across a period and decreases down a group.
Across a period, atomic size decreases, and nuclear attraction increases, making it easier to gain electrons. Thus, non-metals become more reactive.
Example: Fluorine (F) is more reactive than oxygen (O).
Down a group (e.g., Group 17: Halogens), atomic size increases, and it becomes harder for the nucleus to attract electrons. This reduces reactivity.
Example: Fluorine (F) is more reactive than iodine (I).
Most Reactive Non-Metals:
Halogens (Group 17) are the most reactive non-metals because they only need one more electron to complete their outer shell.
Example: Fluorine (F) is the most reactive non-metal.
Examples
Example 1: Hydrogen in Group 1
Hydrogen is positioned at the top of the periodic table in Group 1, which typically contains alkali metals. Although hydrogen is a nonmetal and shares some properties with alkali metals, it is unique due to its one electron in the outer shell. This allows hydrogen to form covalent bonds and participate in various chemical reactions, making it essential in organic compounds and as a fuel source.
Example 2: Alkali Metals (Group 1)
The alkali metals, including lithium (Li), sodium (Na), and potassium (K), are known for their high reactivity and low melting points. They have one valence electron, which they readily lose to form positive ions (cations) during chemical reactions. For instance, sodium reacts vigorously with water to produce sodium hydroxide and hydrogen gas. Their properties highlight the trend of increasing reactivity as you move down the group.
Example 3: Transition Metals (Groups 3-12)
Transition metals, such as iron (Fe), copper (Cu), and gold (Au), are located in the central block of the periodic table. These elements are characterized by their ability to form variable oxidation states and colored compounds. They play critical roles in various applications, such as iron in steel production and copper in electrical wiring, demonstrating the diversity of chemical properties among these elements.
Example 4: Halogens (Group 17)
The halogens, including fluorine (F), chlorine (Cl), and iodine (I), are found in Group 17 of the periodic table. These elements are known for their high reactivity, particularly with alkali metals to form salts. For example, when sodium reacts with chlorine, it produces sodium chloride (table salt). The reactivity of halogens decreases down the group, with fluorine being the most reactive.
Example 5: Noble Gases (Group 18)
The noble gases, such as helium (He), neon (Ne), and argon (Ar), are located in Group 18 of the periodic table. These gases are characterized by their complete valence electron shells, which make them highly stable and nonreactive. Due to their inert nature, noble gases are used in various applications, such as helium in balloons and neon in signs, demonstrating their unique position in the periodic table and their practical uses.
Practice Questions
Question 1
Which of the following correctly describes the organization of the periodic table?
A) Elements are arranged randomly based on their atomic mass.
B) Elements are organized by increasing atomic number and grouped by similar chemical properties.
C) Elements are classified solely based on their physical state (solid, liquid, gas).
D) The periodic table does not provide any information about the reactivity of elements.
Correct Answer: B) Elements are organized by increasing atomic number and grouped by similar chemical properties.
Explanation: The periodic table is systematically organized based on the atomic number, which is the number of protons in an atom's nucleus. Elements with similar chemical properties are placed in the same columns (groups or families), which helps predict their behavior in reactions. Option A is incorrect because the arrangement is not random, option C is misleading as it ignores the importance of atomic number and chemical properties, and option D is false since the table provides valuable information about reactivity.
Question 2
What trend is observed as you move from left to right across a period in the periodic table?
A) Atomic radius increases.
B) Ionization energy decreases.
C) Electronegativity generally increases.
D) Metallic character increases.
Correct Answer: C) Electronegativity generally increases.
Explanation: As you move from left to right across a period, the electronegativity of elements typically increases. This is due to the increasing nuclear charge that attracts electrons more strongly, making atoms better at attracting bonding electrons. Option A is incorrect because atomic radius generally decreases from left to right. Option B is false since ionization energy tends to increase across a period as more energy is required to remove an electron. Option D is also incorrect; metallic character decreases across a period as nonmetals become more prevalent.
Question 3
Which of the following elements is considered a noble gas?
A) Oxygen (O)
B) Nitrogen (N)
C) Argon (Ar)
D) Chlorine (Cl)
Correct Answer: C) Argon (Ar).
Explanation: Noble gases are a group of elements found in Group 18 of the periodic table, characterized by their lack of reactivity due to having a full valence shell of electrons. Argon (Ar) is a noble gas, while oxygen, nitrogen, and chlorine are nonmetals that are found in Groups 16 and 17 and are known for their reactivity. Therefore, option C is the only correct choice as a noble gas.