Proteins are essential macromolecules involved in nearly every biological process, making them a crucial topic for the MCAT. Composed of amino acids linked by peptide bonds, proteins exhibit four levels of structure: primary, secondary, tertiary, and quaternary. They play diverse roles, including enzymatic catalysis, cellular signaling, immune response, structural support, and molecular transport. Examples like hemoglobin, insulin, collagen, and antibodies demonstrate their functional diversity. Understanding protein synthesis, folding, function, and related disorders equips MCAT students with key biochemical knowledge to excel in the exam and medical studies.
Learning Objectives
In studying "Proteins" for the MCAT, you should learn to understand the structure and function of proteins, including primary, secondary, tertiary, and quaternary structures. Analyze key concepts such as enzyme activity, substrate binding, and protein-ligand interactions. Evaluate the roles of proteins in biological processes, including signaling, transport, immune response, and structural support. Explore examples like hemoglobin, insulin, actin, and antibodies to understand their real-world functions. Additionally, study the importance of protein folding, post-translational modifications, and degradation. Apply this knowledge to interpreting biochemical pathways and experimental data in MCAT practice passages, focusing on protein-related concepts in health and disease.
What are proteins ?
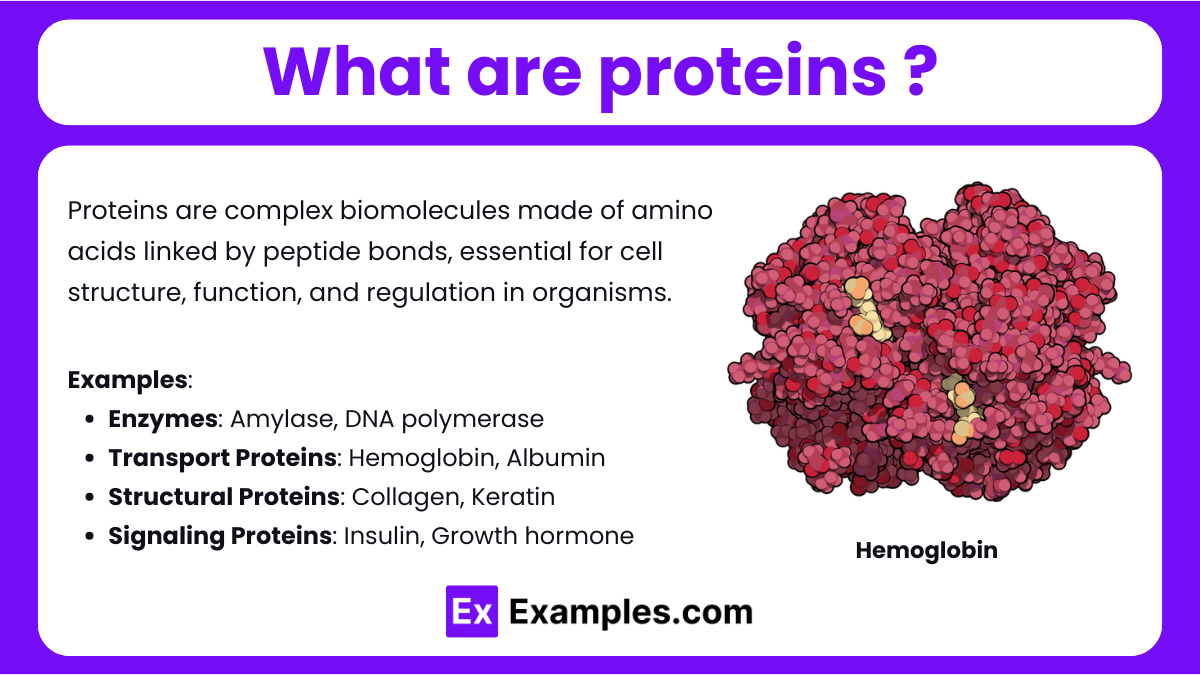
Proteins are large, complex biomolecules composed of long chains of amino acids linked by peptide bonds. They play essential roles in the structure, function, and regulation of cells, tissues, and organs in all living organisms. Proteins perform diverse tasks, such as catalyzing biochemical reactions (as enzymes like amylase and DNA polymerase), transporting molecules (such as hemoglobin and albumin), providing structural support (like collagen and keratin), and facilitating cell signaling and immune responses (such as insulin, growth hormone, and antibodies). Their specific function depends on their unique three-dimensional structure, determined by the sequence of amino acids in the polypeptide chain.
Structure of Proteins
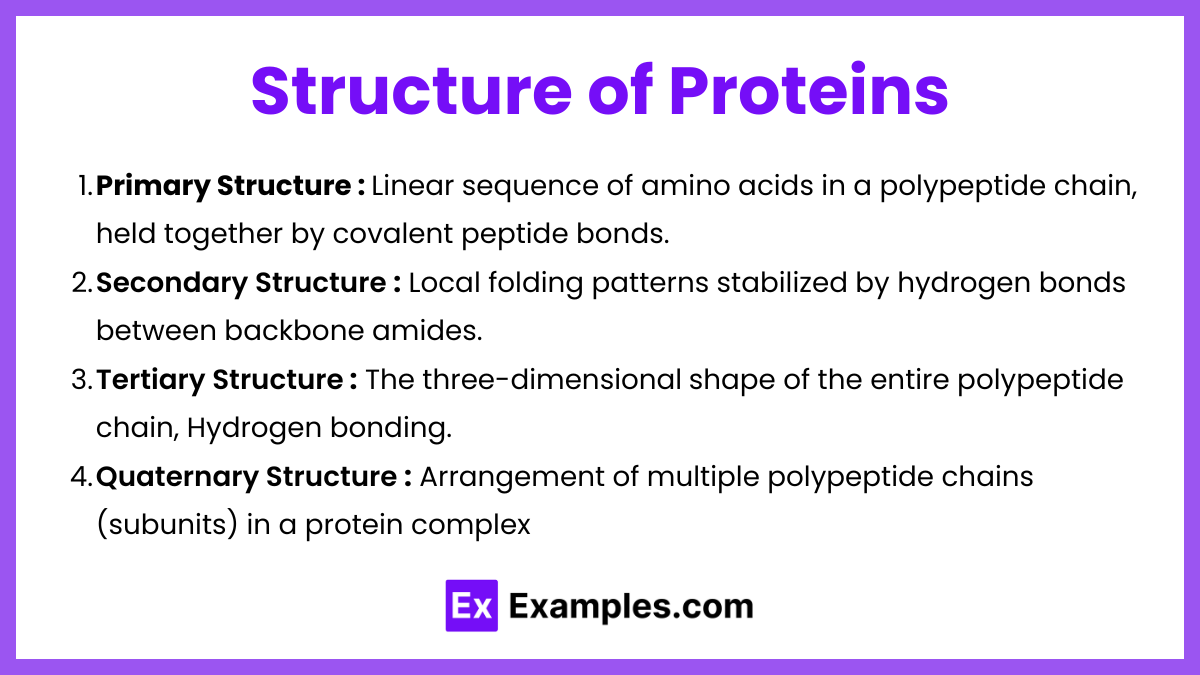
Primary Structure:
Linear sequence of amino acids in a polypeptide chain, held together by covalent peptide bonds.
Example: Glycine-Alanine-Serine-Threonine...
Secondary Structure:
Local folding patterns stabilized by hydrogen bonds between backbone amides.
Alpha-helices: Right-handed coil stabilized by H-bonds (C=O to N-H four residues away).
Beta-sheets: Parallel or antiparallel strands connected by hydrogen bonding.
Tertiary Structure:
The three-dimensional shape of the entire polypeptide chain, involving:
Hydrophobic interactions
Hydrogen bonding
Ionic interactions
Disulfide bonds (covalent bonds between cysteine residues).
Quaternary Structure:
Arrangement of multiple polypeptide chains (subunits) in a protein complex (e.g., hemoglobin has 4 subunits).
Functions of Proteins
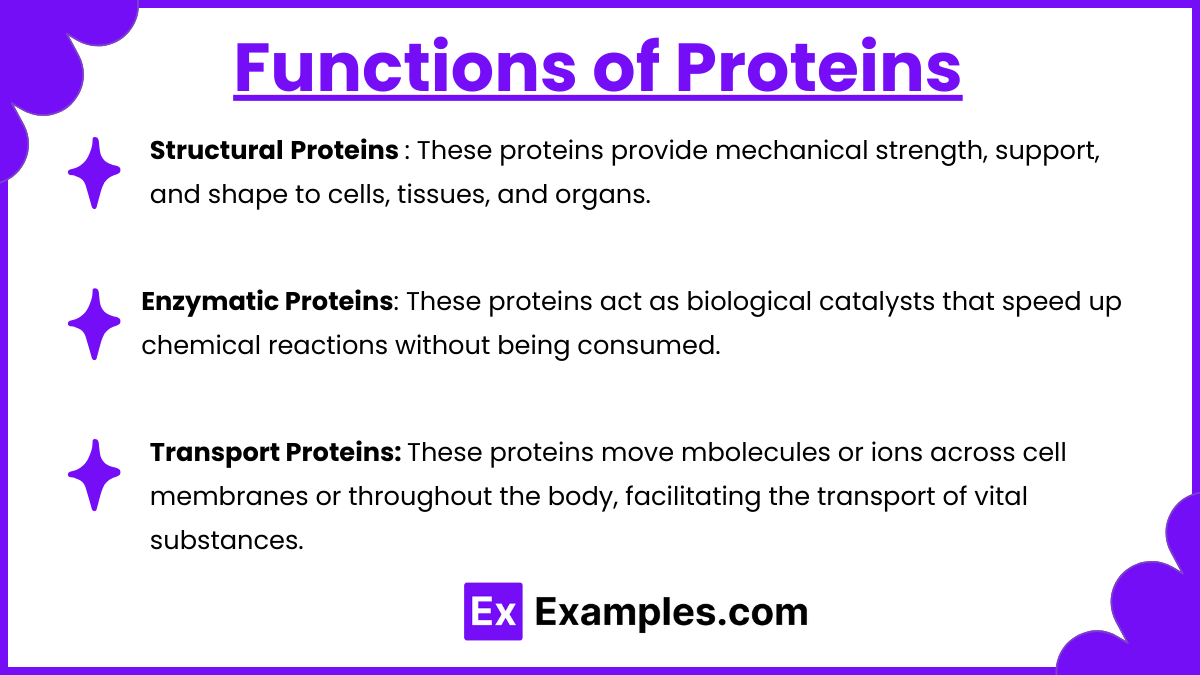
Structural Proteins:These proteins provide mechanical strength, support, and shape to cells, tissues, and organs. They are critical for maintaining the integrity and elasticity of biological structures.
Example: Collagen: Found in connective tissues like tendons and ligaments, providing tensile strength.
Enzymatic Proteins:
These proteins act as biological catalysts that speed up chemical reactions without being consumed. They lower the activation energy, ensuring biochemical processes occur efficiently.Example: DNA Polymerase: Catalyzes the synthesis of DNA during cell division.
Transport Proteins: These proteins move mbolecules or ions across cell membranes or throughout the body, facilitating the transport of vital substances.
Example: Hemoglobin: Binds oxygen in red blood cells and transports it to tissues, while also carrying carbon dioxide back to the lungs.
Signaling Proteins:
These proteins enable communication between cells by transmitting signals that regulate biological processes. They often bind to receptors to initiate specific cellular responses.Example: Insulin: Regulates glucose levels in the blood by promoting glucose uptake in cells.
Defense Proteins:
These proteins protect the body from infections and foreign invaders by recognizing and neutralizing pathogens. They play a crucial role in the immune system.Example: Antibodies (Immunoglobulins): Bind specifically to antigens on pathogens to neutralize them.
Protein Synthesis and Degradation
Protein Synthesis : Protein synthesis is the biological process by which cells produce proteins. It involves two main stages: transcription and translation.
Transcription: DNA → mRNA in the nucleus.
Translation: mRNA → Protein at the ribosome in the cytoplasm.
Initiation: Ribosome binds to the mRNA.
Elongation: Amino acids are added sequentially by tRNA.
Termination: Ribosome reaches a stop codon, and the polypeptide chain is released.
Protein Folding and Chaperones
Chaperone proteins assist in the proper folding of newly synthesized proteins.
Misfolded proteins can lead to diseases such as Alzheimer’s or Parkinson’s.
Protein Degradation
Ubiquitin-Proteasome Pathway: Marks proteins for degradation by tagging them with ubiquitin.
Autophagy: Degrades misfolded proteins and damaged organelles.
Examples
Examples of Proteins for the MCAT
Example 1: Hemoglobin
Hemoglobin is a transport protein found in red blood cells, responsible for carrying oxygen from the lungs to tissues throughout the body and transporting carbon dioxide back to the lungs for exhalation. It exhibits cooperativity, meaning the binding of oxygen to one subunit increases the affinity of the other subunits for oxygen. Mutations in hemoglobin can lead to conditions like sickle cell anemia.
Example 2 : Collagen
Collagen is a structural protein that provides tensile strength and support to connective tissues, such as skin, cartilage, bones, and tendons. It makes up around 30% of the total protein in the human body. Collagen molecules form a triple helix structure, giving them remarkable strength. Disorders in collagen synthesis can result in diseases like Ehlers-Danlos syndrome or osteogenesis imperfecta.
Example 3: Insulin
Insulin is a peptide hormone and signaling protein secreted by the pancreas in response to elevated blood glucose levels. It plays a crucial role in glucose metabolism by facilitating the uptake of glucose into cells and promoting glycogen storage in the liver. Insufficient insulin production or cellular insensitivity to insulin causes diabetes mellitus, which is a major topic in the MCAT.
Example 4 : Actin
Actin is a key structural protein involved in cell shape, motility, and intracellular transport. It forms microfilaments, which are part of the cytoskeleton. Actin plays an essential role in muscle contraction, where it interacts with myosin, another motor protein. Disruptions in actin filaments can lead to impaired cell movement and diseases such as congenital myopathy.
Example 5 : Antibodies (Immunoglobulins)
Antibodies are immune proteins produced by B cells to recognize and neutralize pathogens like bacteria and viruses. Each antibody has a specific binding site that matches an antigen, making them highly selective. They play a pivotal role in both innate and adaptive immune responses, and their function is heavily tested on the MCAT, especially in questions related to immunity and vaccine mechanisms.
Practice Questions
Question 1
Which of the following interactions stabilizes the tertiary structure of proteins?
A) Peptide bonds
B) Phosphodiester bonds
C) Hydrogen bonds, ionic bonds, and hydrophobic interactions
D) Glycosidic bonds
Answer: C) Hydrogen bonds, ionic bonds, and hydrophobic interactions
Explanation: The tertiary structure of proteins refers to the overall 3D folding of a polypeptide chain. This structure is stabilized by several types of interactions, including hydrogen bonds, ionic bonds (between charged side chains), and hydrophobic interactions (involving non-polar side chains). While peptide bonds (Option A) form the backbone of the primary structure, they do not contribute directly to the tertiary structure. Phosphodiester bonds (Option B) are found in nucleic acids, not proteins. Glycosidic bonds (Option D) are involved in carbohydrates, not proteins. Thus, the correct answer is C.
Question 2
Which of the following proteins is responsible for transporting oxygen in the bloodstream?
A) Myosin
B) Hemoglobin
C) Collagen
D) Actin
Answer: B) Hemoglobin
Explanation: Hemoglobin is a globular protein found in red blood cells, where it binds oxygen in the lungs and transports it throughout the body. Each hemoglobin molecule can carry up to four oxygen molecules. Myosin (Option A) is involved in muscle contraction, collagen (Option C) provides structural support in connective tissues, and actin (Option D) plays a role in cell motility and structure. Therefore, the correct answer is B
Question 3
Which of the following statements about enzymes is false?
A) Enzymes increase the activation energy of a reaction.
B) Enzymes act as biological catalysts.
C) Enzymes are specific to their substrates.
D) Enzymes lower the activation energy required for a reaction to occur.
Answer: A) Enzymes increase the activation energy of a reaction
Explanation: Enzymes function as biological catalysts by lowering the activation energy required for chemical reactions to proceed, which makes reactions occur faster. Option A is false because enzymes reduce, not increase, activation energy. Enzymes are specific to their substrates (Option C), meaning they catalyze only specific reactions. The other options correctly describe enzymatic function, so the false statement, and the correct answer, is A.