Solubility equilibria

- Notes
Solubility equilibria describe the balance between dissolved ions and undissolved solids in a saturated solution. Key concepts include the solubility product constant $ K_{sp} $, common ion effect, and predicting precipitation using $ Q $ versus $ K_{sp} $. These principles are vital in biological systems and analytical chemistry.
Learning Objectives
In studying Solubility Equilibria for the MCAT, you should understand how saturated solutions achieve dynamic equilibrium, calculate solubility from $ K_{sp} $, and analyze the effects of the common ion effect on solubility. You should also be able to differentiate between the reaction quotient $ Q $ and $ K_{sp} $ to predict precipitation, and apply these principles to real-world biological and chemical processes, such as kidney stone formation and blood plasma ion balance.
What is Solubility Equilibria?
Solubility equilibria refer to the balance established between the dissolved ions and the undissolved solid in a saturated solution. The concept describes the equilibrium that exists when a solute’s dissolution and precipitation occur at the same rate, leading to a constant concentration of ions in solution.
Saturated Solutions and Solubility Product ( Ksp )
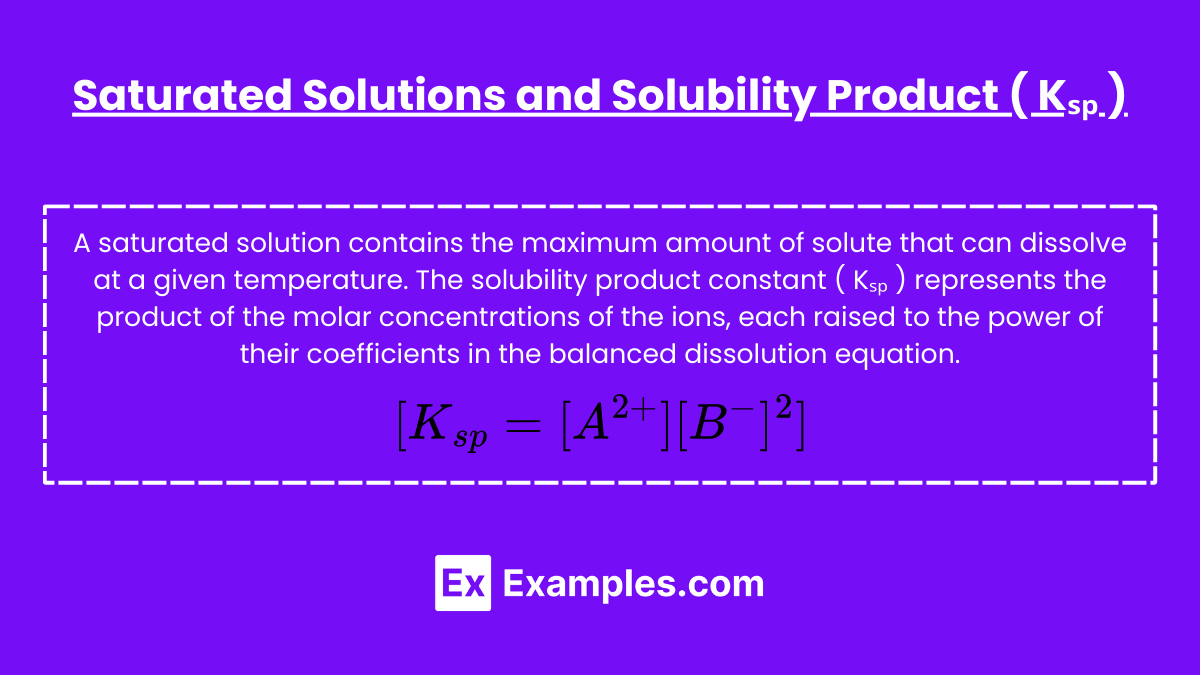
A saturated solution contains the maximum amount of solute that can dissolve at a given temperature. The solubility product constant ( Kₛₚ ) represents the product of the molar concentrations of the ions, each raised to the power of their coefficients in the balanced dissolution equation.
For a generic salt ( AB₂ ), the dissociation can be represented as:
$[AB_2 \leftrightarrow A^{2+} + 2B^-]$
The expression for the solubility product constant ($K_{sp}$) is:
$[K_{sp} = [A^{2+}][B^-]^2]$
The value of ($K_{sp}$) is specific to each solute and indicates its solubility at a given temperature.
Common Ion Effect on Solubility
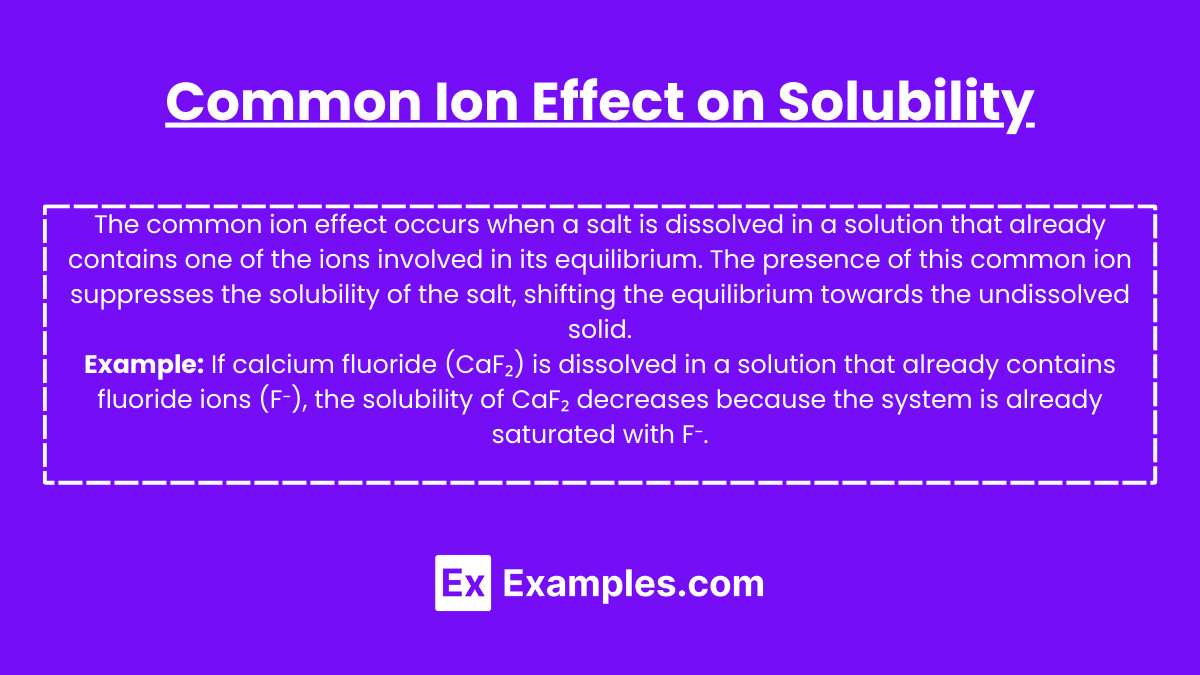
The common ion effect occurs when a salt is dissolved in a solution that already contains one of the ions involved in its equilibrium. The presence of this common ion suppresses the solubility of the salt, shifting the equilibrium towards the undissolved solid.
Example: If calcium fluoride (CaF2) is dissolved in a solution that already contains fluoride ions (F–), the solubility of CaF2 decreases because the system is already saturated with F–.
Applications of Solubility Equilibria in Biology and Medicine
- Kidney Stones: Calcium oxalate and uric acid stones form when the concentration of these salts exceeds their solubility in urine, leading to precipitation.
- Blood Plasma: Solubility equilibria govern the concentrations of ions in blood plasma, such as calcium and phosphate, which are critical for maintaining proper physiological functions.
Precipitation and Q vs. Ksp
The reaction quotient ($Q$) is used to determine whether a precipitate will form in a solution:
If $Q > K_{sp}$: A precipitate forms, as the solution is supersaturated.
If $Q < K_{sp}$: No precipitate forms, as the solution is unsaturated.
If $Q = K_{sp}$: The solution is exactly saturated, and no change occurs.
Examples
Example 1: Silver Chloride Solubility
Silver chloride ($ \text{AgCl} $) is a sparingly soluble salt. Its solubility equilibrium is described by $ \text{AgCl} \leftrightarrow \text{Ag}^+ + \text{Cl}^- $, and its solubility product constant $ K_{sp} $ is $ 1.8 \times 10^{-10} $.
Example 2: Calcium Fluoride and Common Ion Effect
In the presence of sodium fluoride ($ \text{NaF} $), the solubility of calcium fluoride ($ \text{CaF}_2 $) decreases due to the common ion effect. $ \text{CaF}_2 \leftrightarrow \text{Ca}^{2+} + 2\text{F}^- $, and the additional fluoride ions shift the equilibrium toward the undissolved salt.
Example 3: Precipitation of Barium Sulfate
Selective precipitation separates metal ions based on their differing solubility products. For example, adding sulfate ions to a mixture of barium and magnesium ions causes barium to precipitate first as (BaSO₄) , which has a lower solubility product (Kₛₚ) than magnesium sulfate.
Example 4: Reducing Solubility with a Common Ion
The solubility of $ \text{PbCl}_2 $ decreases when dissolved in a solution containing $ \text{Cl}^- $ ions, due to the common ion effect. The equilibrium $ \text{PbCl}_2 \leftrightarrow \text{Pb}^{2+} + 2\text{Cl}^- $ shifts left, reducing lead ion concentration.
Example 5: Selective Precipitation in Metal Ion Separation
Selective precipitation separates metal ions based on their differing solubility products. For example, adding sulfate ions to a mixture of barium and magnesium ions causes barium to precipitate first as (BaSO₄), which has a lower (Kₛₚ) than magnesium sulfate.
Practice Questions
Question 1:
The $ K_{sp} $ of $ \text{CaF}_2 $ is $ 3.9 \times 10^{-11} $. What is the molar solubility of $ \text{CaF}_2 $ in water?
A) $ 1.5 \times 10^{-4} , \text{mol/L} $
B) $ 3.9 \times 10^{-11} , \text{mol/L} $
C) $ 2.1 \times 10^{-4} , \text{mol/L} $
D) $ 6.3 \times 10^{-6} , \text{mol/L} $
Answer:
A) $ 1.5 \times 10^{-4} , \text{mol/L} $
Explanation:
For $ \text{CaF}_2 $, the dissociation is $ \text{CaF}_2 \leftrightarrow \text{Ca}^{2+} + 2\text{F}^- $. Let $ s $ represent the molar solubility:
The solubility product constant ($K_{sp}$) for calcium fluoride ($CaF_2$) can be expressed as:
$K_{sp} = [Ca^{2+}][F^-]^2 = s(2s)^2 = 4s^3$
Given $K_{sp} = 3.9 \times 10^{-11}$, we solve for $s$:
$3.9 \times 10^{-11} = 4s^3 \implies s = \sqrt[3]{\frac{3.9 \times 10^{-11}}{4}} = 1.5 \times 10^{-4} \, \text{mol/L}$
Question 2:
What happens to the solubility of $ \text{AgCl} $ when NaCl is added to the solution?
A) Solubility increases
B) Solubility decreases
C) Solubility remains unchanged
D) Precipitation occurs
Answer:
B) Solubility decreases
Explanation:
Adding NaCl introduces $ \text{Cl}^- $ ions, which is a common ion. This increases the concentration of $ \text{Cl}^- $ in solution, shifting the equilibrium $ \text{AgCl} \leftrightarrow \text{Ag}^+ + \text{Cl}^- $ towards the undissolved $ \text{AgCl} $, reducing solubility due to the common ion effect.
Question 3:
If the $ K_{sp} $ of lead(II) sulfate ($ \text{PbSO}_4 $) is $ 1.6 \times 10^{-8} $, what is the concentration of $ \text{SO}_4^{2-} $ in a saturated solution?
A) $ 4.0 \times 10^{-4} , \text{mol/L} $
B) $ 1.6 \times 10^{-8} , \text{mol/L} $
C) $ 1.3 \times 10^{-4} , \text{mol/L} $
D) $ 2.5 \times 10^{-4} , \text{mol/L} $
Answer:
C) $ 1.3 \times 10^{-4} , \text{mol/L} $
Explanation:
For $ \text{PbSO}_4 $, the dissociation is $ \text{PbSO}_4 \leftrightarrow \text{Pb}^{2+} + \text{SO}_4^{2-} $. Let $ s $ represent the molar solubility:
The solubility product constant ($K_{sp}$) for lead sulfate ($PbSO_4$) can be expressed as:
$K_{sp} = [Pb^{2+}][SO_4^{2-}] = s^2$
Given $K_{sp} = 1.6 \times 10^{-8}$, we solve for $s$:
$s = \sqrt{K_{sp}} = \sqrt{1.6 \times 10^{-8}} = 1.3 \times 10^{-4} \, \text{mol/L}$
Thus, the concentration of sulfate ions is $ 1.3 \times 10^{-4} , \text{mol/L} $.
Solubility equilibria describe the balance between dissolved ions and undissolved solids in a saturated solution. Key concepts include the solubility product constant Ksp$ K_{sp} $, common ion effect, and predicting precipitation using Q$ Q $ versus Ksp$ K_{sp} $. These principles are vital in biological systems and analytical chemistry.
Learning Objectives
In studying Solubility Equilibria for the MCAT, you should understand how saturated solutions achieve dynamic equilibrium, calculate solubility from Ksp$ K_{sp} $, and analyze the effects of the common ion effect on solubility. You should also be able to differentiate between the reaction quotient Q$ Q $ and Ksp$ K_{sp} $ to predict precipitation, and apply these principles to real-world biological and chemical processes, such as kidney stone formation and blood plasma ion balance.
What is Solubility Equilibria?
Solubility equilibria refer to the balance established between the dissolved ions and the undissolved solid in a saturated solution. The concept describes the equilibrium that exists when a solute's dissolution and precipitation occur at the same rate, leading to a constant concentration of ions in solution.
Saturated Solutions and Solubility Product ( Ksp )
A saturated solution contains the maximum amount of solute that can dissolve at a given temperature. The solubility product constant ( Kₛₚ ) represents the product of the molar concentrations of the ions, each raised to the power of their coefficients in the balanced dissolution equation.
For a generic salt ( AB₂ ), the dissociation can be represented as:
[AB2↔A2++2B−]$[AB_2 \leftrightarrow A^{2+} + 2B^-]$
The expression for the solubility product constant (Ksp$K_{sp}$) is:
[Ksp=[A2+][B−]2]$[K_{sp} = [A^{2+}][B^-]^2]$
The value of (Ksp$K_{sp}$) is specific to each solute and indicates its solubility at a given temperature.
Common Ion Effect on Solubility
The common ion effect occurs when a salt is dissolved in a solution that already contains one of the ions involved in its equilibrium. The presence of this common ion suppresses the solubility of the salt, shifting the equilibrium towards the undissolved solid.
Example: If calcium fluoride (CaF2) is dissolved in a solution that already contains fluoride ions (F-), the solubility of CaF2 decreases because the system is already saturated with F-.
Applications of Solubility Equilibria in Biology and Medicine
Kidney Stones: Calcium oxalate and uric acid stones form when the concentration of these salts exceeds their solubility in urine, leading to precipitation.
Blood Plasma: Solubility equilibria govern the concentrations of ions in blood plasma, such as calcium and phosphate, which are critical for maintaining proper physiological functions.
Precipitation and Q vs. Ksp
The reaction quotient (Q$Q$) is used to determine whether a precipitate will form in a solution:
If Q>Ksp$Q > K_{sp}$: A precipitate forms, as the solution is supersaturated.
If Q<Ksp$Q < K_{sp}$: No precipitate forms, as the solution is unsaturated.
If Q=Ksp$Q = K_{sp}$: The solution is exactly saturated, and no change occurs.
Examples
Example 1: Silver Chloride Solubility
Silver chloride (AgCl$ \text{AgCl} $) is a sparingly soluble salt. Its solubility equilibrium is described by AgCl↔Ag++Cl−$ \text{AgCl} \leftrightarrow \text{Ag}^+ + \text{Cl}^- $, and its solubility product constant Ksp$ K_{sp} $ is 1.8×10−10$ 1.8 \times 10^{-10} $.
Example 2: Calcium Fluoride and Common Ion Effect
In the presence of sodium fluoride (NaF$ \text{NaF} $), the solubility of calcium fluoride (CaF2$ \text{CaF}_2 $) decreases due to the common ion effect. CaF2↔Ca2++2F−$ \text{CaF}_2 \leftrightarrow \text{Ca}^{2+} + 2\text{F}^- $, and the additional fluoride ions shift the equilibrium toward the undissolved salt.
Example 3: Precipitation of Barium Sulfate
Selective precipitation separates metal ions based on their differing solubility products. For example, adding sulfate ions to a mixture of barium and magnesium ions causes barium to precipitate first as (BaSO₄) , which has a lower solubility product (Kₛₚ) than magnesium sulfate.
Example 4: Reducing Solubility with a Common Ion
The solubility of PbCl2$ \text{PbCl}_2 $ decreases when dissolved in a solution containing Cl−$ \text{Cl}^- $ ions, due to the common ion effect. The equilibrium PbCl2↔Pb2++2Cl−$ \text{PbCl}_2 \leftrightarrow \text{Pb}^{2+} + 2\text{Cl}^- $ shifts left, reducing lead ion concentration.
Example 5: Selective Precipitation in Metal Ion Separation
Selective precipitation separates metal ions based on their differing solubility products. For example, adding sulfate ions to a mixture of barium and magnesium ions causes barium to precipitate first as (BaSO₄), which has a lower (Kₛₚ) than magnesium sulfate.
Practice Questions
Question 1:
The Ksp$ K_{sp} $ of CaF2$ \text{CaF}_2 $ is 3.9×10−11$ 3.9 \times 10^{-11} $. What is the molar solubility of CaF2$ \text{CaF}_2 $ in water?
A) 1.5×10−4,mol/L$ 1.5 \times 10^{-4} , \text{mol/L} $
B) 3.9×10−11,mol/L$ 3.9 \times 10^{-11} , \text{mol/L} $
C) 2.1×10−4,mol/L$ 2.1 \times 10^{-4} , \text{mol/L} $
D) 6.3×10−6,mol/L$ 6.3 \times 10^{-6} , \text{mol/L} $
Answer:
A) 1.5×10−4,mol/L$ 1.5 \times 10^{-4} , \text{mol/L} $
Explanation:
For CaF2$ \text{CaF}_2 $, the dissociation is CaF2↔Ca2++2F−$ \text{CaF}_2 \leftrightarrow \text{Ca}^{2+} + 2\text{F}^- $. Let s$ s $ represent the molar solubility:
The solubility product constant (Ksp$K_{sp}$) for calcium fluoride (CaF2$CaF_2$) can be expressed as:
Ksp=[Ca2+][F−]2=s(2s)2=4s3$K_{sp} = [Ca^{2+}][F^-]^2 = s(2s)^2 = 4s^3$
Given Ksp=3.9×10−11$K_{sp} = 3.9 \times 10^{-11}$, we solve for s$s$:
3.9×10−11=4s3⟹s=343.9×10−11=1.5×10−4mol/L$3.9 \times 10^{-11} = 4s^3 \implies s = \sqrt[3]{\frac{3.9 \times 10^{-11}}{4}} = 1.5 \times 10^{-4} \, \text{mol/L}$
Question 2:
What happens to the solubility of AgCl$ \text{AgCl} $ when NaCl is added to the solution?
A) Solubility increases
B) Solubility decreases
C) Solubility remains unchanged
D) Precipitation occurs
Answer:
B) Solubility decreases
Explanation:
Adding NaCl introduces Cl−$ \text{Cl}^- $ ions, which is a common ion. This increases the concentration of Cl−$ \text{Cl}^- $ in solution, shifting the equilibrium AgCl↔Ag++Cl−$ \text{AgCl} \leftrightarrow \text{Ag}^+ + \text{Cl}^- $ towards the undissolved AgCl$ \text{AgCl} $, reducing solubility due to the common ion effect.
Question 3:
If the Ksp$ K_{sp} $ of lead(II) sulfate (PbSO4$ \text{PbSO}_4 $) is 1.6×10−8$ 1.6 \times 10^{-8} $, what is the concentration of SO42−$ \text{SO}_4^{2-} $ in a saturated solution?
A) 4.0×10−4,mol/L$ 4.0 \times 10^{-4} , \text{mol/L} $
B) 1.6×10−8,mol/L$ 1.6 \times 10^{-8} , \text{mol/L} $
C) 1.3×10−4,mol/L$ 1.3 \times 10^{-4} , \text{mol/L} $
D) 2.5×10−4,mol/L$ 2.5 \times 10^{-4} , \text{mol/L} $
Answer:
C) 1.3×10−4,mol/L$ 1.3 \times 10^{-4} , \text{mol/L} $
Explanation:
For PbSO4$ \text{PbSO}_4 $, the dissociation is PbSO4↔Pb2++SO42−$ \text{PbSO}_4 \leftrightarrow \text{Pb}^{2+} + \text{SO}_4^{2-} $. Let s$ s $ represent the molar solubility:
The solubility product constant (Ksp$K_{sp}$) for lead sulfate (PbSO4$PbSO_4$) can be expressed as:
Ksp=[Pb2+][SO42−]=s2$K_{sp} = [Pb^{2+}][SO_4^{2-}] = s^2$
Given Ksp=1.6×10−8$K_{sp} = 1.6 \times 10^{-8}$, we solve for s$s$:
s=Ksp=1.6×10−8=1.3×10−4mol/L$s = \sqrt{K_{sp}} = \sqrt{1.6 \times 10^{-8}} = 1.3 \times 10^{-4} \, \text{mol/L}$
Thus, the concentration of sulfate ions is 1.3×10−4,mol/L$ 1.3 \times 10^{-4} , \text{mol/L} $.