The Fluid, Electrolyte, and Acid-Base Balance section of the NCLEX-RN® exam assesses a nurse's understanding of critical physiological processes essential for maintaining homeostasis. This includes the regulation of fluid distribution, electrolyte levels (such as sodium, potassium, calcium, and magnesium), and acid-base balance through mechanisms like respiratory and renal systems. Nurses must identify imbalances like dehydration, hyperkalemia, or metabolic acidosis, understand their clinical manifestations, and prioritize interventions. Mastery of this topic is vital for safe and effective nursing care in various patient scenarios.
Learning Objectives
In studying "Adult Health: Fluid, Electrolyte, Acid-Base Balance" for the NCLEX RN® exam, you should learn to understand the principles of fluid distribution, electrolyte balance, and acid-base homeostasis. Analyze common imbalances such as dehydration, hypervolemia, hyponatremia, and metabolic acidosis, and evaluate their clinical manifestations and treatment protocols. Understand key laboratory values, including serum electrolytes, arterial blood gases (ABGs), and osmolality, and how they guide nursing interventions. Additionally, explore the role of isotonic, hypertonic, and hypotonic fluids in restoring balance and apply your knowledge to interpreting patient scenarios and prioritizing care for effective management of these critical health conditions.
Fluid Balance

Fluid balance refers to the state in which the amount of water and electrolytes entering the body (through oral intake, intravenous fluids, and food) is equal to the amount being lost (through urine, sweat, respiration, and stool). Maintaining fluid balance is essential for homeostasis, ensuring proper cellular function, circulation, and overall health.
Key Concepts:
Body Fluid Compartments:
Intracellular (ICF): ~2/3 of body water.
Extracellular (ECF): Interstitial and intravascular compartments.
Fluid Movement Mechanisms:
Osmosis: Movement of water across semipermeable membranes.
Diffusion: Movement of solutes.
Filtration: Movement due to hydrostatic pressure.
Active Transport: Energy-driven solute movement (e.g., sodium-potassium pump).
Types of Fluids:
Isotonic: 0.9% NS, LR (used for volume replacement).
Hypotonic: 0.45% NS (shifts fluid into cells).
Hypertonic: 3% NS (shifts fluid out of cells).
Nursing Implications:
Monitor intake and output (I&O).
Assess for signs of dehydration (dry mucous membranes, poor skin turgor, tachycardia) and fluid overload (edema, jugular vein distention, crackles).
Electrolyte Balance
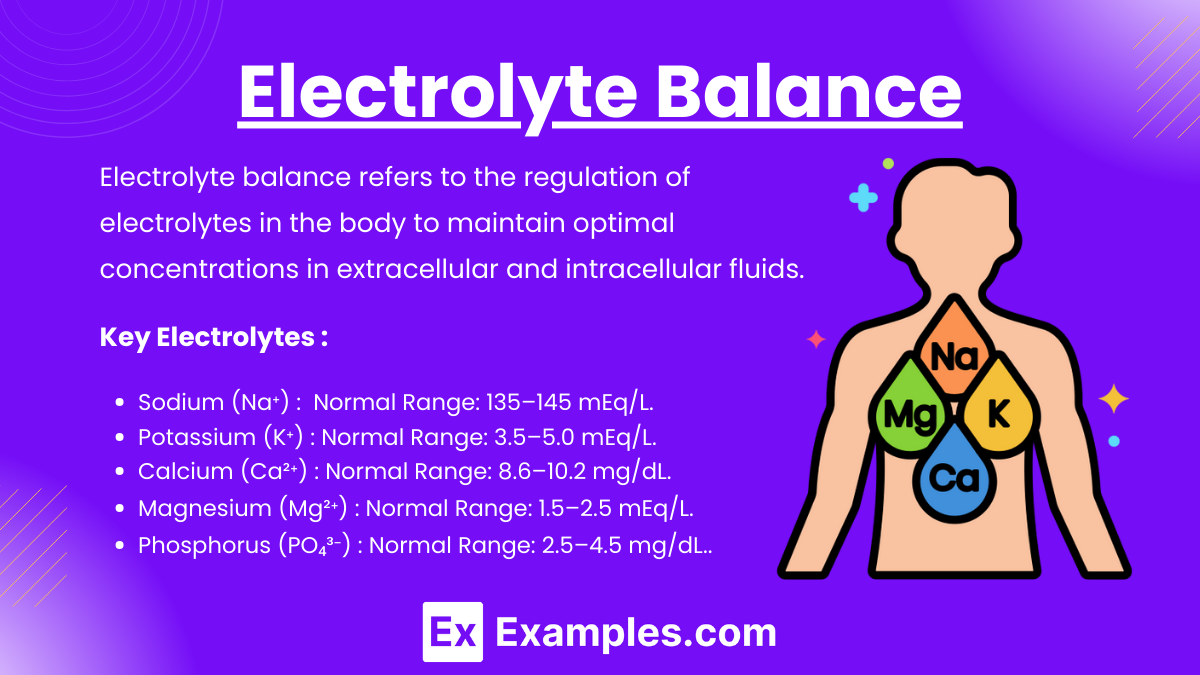
Electrolyte balance refers to the regulation of electrolytes in the body to maintain optimal concentrations in extracellular and intracellular fluids. Electrolytes, such as sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), magnesium (Mg²⁺), chloride (Cl⁻), bicarbonate (HCO₃⁻), and phosphate (PO₄³⁻), are minerals that carry an electrical charge and are essential for physiological functions, including:
Key Electrolytes:
Sodium (Na⁺):
Normal Range: 135–145 mEq/L.
Hyponatremia: Causes confusion, seizures.
Hypernatremia: Causes restlessness, dehydration.
Potassium (K⁺):
Normal Range: 3.5–5.0 mEq/L.
Hypokalemia: Weakness, arrhythmias (monitor ECG).
Hyperkalemia: Muscle cramps, peaked T waves (treat with insulin and glucose).
Calcium (Ca²⁺):
Normal Range: 8.6–10.2 mg/dL.
Hypocalcemia: Tetany, Chvostek's and Trousseau's signs.
Hypercalcemia: Weakness, kidney stones.
Magnesium (Mg²⁺):
Normal Range: 1.5–2.5 mEq/L.
Hypomagnesemia: Tremors, hyperreflexia.
Hypermagnesemia: Respiratory depression, bradycardia.
Phosphorus (PO₄³⁻):
Normal Range: 2.5–4.5 mg/dL.
Inverse relationship with calcium.
Nursing Implications:
Correct electrolyte imbalances cautiously to prevent iatrogenic complications.
Monitor cardiac and neuromuscular function.
Acid-Base Balance
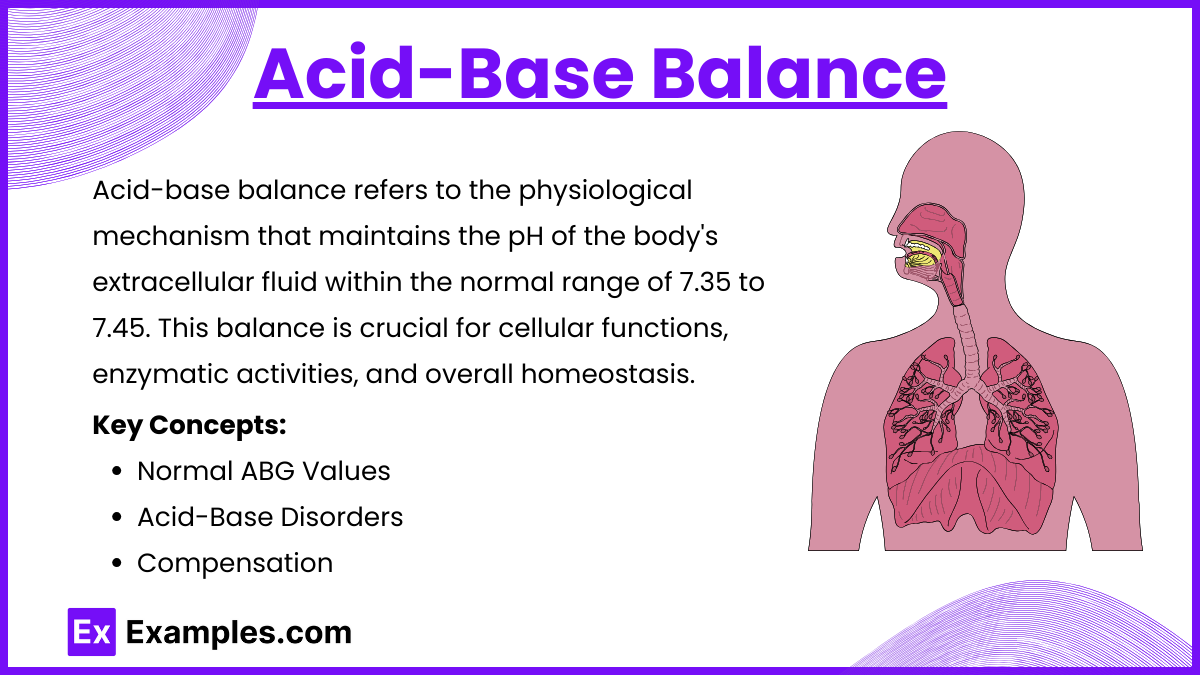
Acid-base balance refers to the physiological mechanism that maintains the pH of the body's extracellular fluid within the normal range of 7.35 to 7.45. This balance is crucial for cellular functions, enzymatic activities, and overall homeostasis.
Key Concepts:
Normal ABG Values:
pH: 7.35–7.45.
PaCO₂: 35–45 mmHg.
HCO₃⁻: 22–26 mEq/L.
Acid-Base Disorders:
Respiratory Acidosis: Hypoventilation, CO₂ retention.
Respiratory Alkalosis: Hyperventilation, CO₂ loss.
Metabolic Acidosis: Decreased HCO₃⁻ (e.g., diarrhea).
Metabolic Alkalosis: Increased HCO₃⁻ (e.g., vomiting).
Compensation:
Lungs compensate for metabolic imbalances.
Kidneys compensate for respiratory imbalances.
Nursing Implications:
Identify causative factors (e.g., renal failure, COPD, DKA).
Monitor ABGs for treatment effectiveness (e.g., oxygen therapy, bicarbonate).
Common Disorders and Management
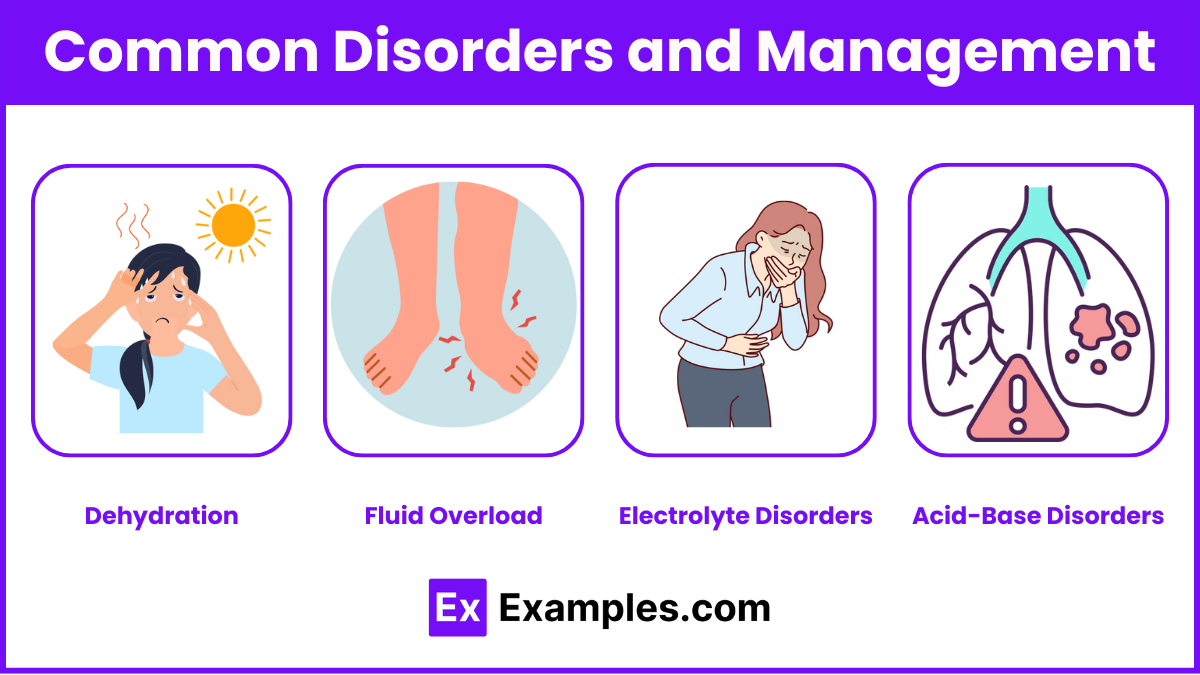
Dehydration:
Signs: Tachycardia, hypotension, dark urine.
Management: Isotonic fluids, electrolyte replacement.
Fluid Overload:
Signs: Edema, crackles, increased BP.
Management: Diuretics, fluid restriction.
Electrolyte Disorders:
Administer appropriate supplements or treatments cautiously.
Use telemetry for potassium imbalances.
Acid-Base Disorders:
Treat underlying cause (e.g., administer bronchodilators for respiratory acidosis).
Examples
Example 1: Dehydration and Hypovolemia
A 65-year-old patient presents with complaints of dizziness, dry mouth, and decreased urine output. Vital signs reveal hypotension, tachycardia, and orthostatic hypotension. Laboratory results show elevated blood urea nitrogen (BUN) and hematocrit levels, indicating dehydration. The nurse’s priority intervention includes administering isotonic fluids (e.g., 0.9% normal saline) to restore intravascular volume. Education focuses on increasing oral fluid intake and recognizing signs of dehydration, ensuring long-term prevention.
Example 2: Hyperkalemia in Renal Failure
A patient with chronic kidney disease (CKD) arrives at the emergency department with complaints of muscle weakness and palpitations. An electrocardiogram (ECG) shows peaked T waves. Laboratory values confirm potassium levels of 6.5 mEq/L. The nurse prepares to administer calcium gluconate to stabilize the cardiac membrane, followed by insulin and glucose to shift potassium into cells. Sodium polystyrene sulfonate (Kayexalate) may be given to facilitate potassium excretion. Ongoing monitoring of potassium levels and cardiac rhythm is crucial.
Example 3:Metabolic Acidosis in Diabetic Ketoacidosis (DKA)
A 45-year-old patient with type 1 diabetes is admitted with symptoms of rapid breathing (Kussmaul respirations), fruity breath odor, and confusion. Arterial blood gas (ABG) results reveal a pH of 7.25, HCO₃⁻ of 15 mEq/L, and blood glucose over 600 mg/dL. These findings indicate metabolic acidosis due to DKA. The nurse initiates IV insulin therapy to reduce blood glucose, administers fluids to correct dehydration, and monitors potassium levels closely, as insulin therapy can cause hypokalemia.
Example 4: Hyponatremia in Syndrome of Inappropriate Antidiuretic Hormone (SIADH)
A patient with a history of small-cell lung cancer presents with confusion, nausea, and muscle cramps. Serum sodium levels are 120 mEq/L, and urine is concentrated with high osmolality. The diagnosis of SIADH is confirmed. The nurse administers hypertonic saline (e.g., 3% NaCl) cautiously, monitoring for signs of cerebral edema and fluid overload. Fluid restriction and electrolyte monitoring are emphasized as part of the ongoing care plan.
Example 5: Respiratory Acidosis in Chronic Obstructive Pulmonary Disease (COPD)
A 70-year-old patient with COPD exhibits signs of dyspnea, cyanosis, and drowsiness. ABG analysis shows a pH of 7.30, PaCO₂ of 60 mmHg, and HCO₃⁻ of 28 mEq/L, indicating uncompensated respiratory acidosis. The nurse administers low-flow oxygen therapy to improve oxygenation while avoiding suppression of the hypoxic drive. Bronchodilators and corticosteroids are given to relieve airway obstruction. Patient education includes techniques to improve breathing efficiency, such as pursed-lip breathing.
Practice Questions
Question 1
A patient with Type 1 Diabetes Mellitus is admitted for diabetic ketoacidosis (DKA). Which of the following lab findings is most indicative of DKA?
A. Blood glucose of 120 mg/dL
B. Serum pH of 7.30
C. Potassium level of 5.2 mEq/L
D. Serum bicarbonate of 24 mEq/L
Answer: B. Serum pH of 7.30
Explanation:
DKA is characterized by metabolic acidosis, with low pH (<7.35), hyperglycemia, and ketone production.
A (Blood glucose of 120 mg/dL): Incorrect. DKA is associated with hyperglycemia, typically >250 mg/dL.
B (Serum pH of 7.30): Correct. Acidosis is a hallmark of DKA, indicated by pH <7.35.
C (Potassium level of 5.2 mEq/L): Incorrect. Potassium may be elevated in DKA due to a shift of potassium out of cells, but it is not diagnostic.
D (Serum bicarbonate of 24 mEq/L): Incorrect. DKA involves decreased bicarbonate (<18 mEq/L) due to buffering of ketone acids.
Question 2
A nurse is caring for a patient with hyperthyroidism. Which clinical manifestation would the nurse expect to find?
A. Bradycardia
B. Cold intolerance
C. Weight gain
D. Exophthalmos
Answer: D. Exophthalmos
Explanation:
Hyperthyroidism (e.g., in Graves' disease) causes an overproduction of thyroid hormones, increasing metabolism.
A (Bradycardia): Incorrect. Tachycardia is more typical due to increased metabolic rate.
B (Cold intolerance): Incorrect. Heat intolerance is common due to excessive metabolism.
C (Weight gain): Incorrect. Weight loss occurs despite increased appetite.
D (Exophthalmos): Correct. Exophthalmos (bulging eyes) is a classic sign of hyperthyroidism, particularly Graves' disease, due to immune-mediated tissue swelling.
Question 3
A patient with Addison's disease is admitted to the hospital with severe hypotension and weakness. What is the priority nursing intervention?
A. Administer insulin and dextrose
B. Administer IV hydrocortisone
C. Restrict sodium intake
D. Monitor for signs of infection
Answer: B. Administer IV hydrocortisone
Explanation:
Addison's disease (adrenal insufficiency) leads to deficient cortisol and aldosterone levels. In an Addisonian crisis, IV hydrocortisone is the priority to replace deficient cortisol.
A (Administer insulin and dextrose): Incorrect. This is for hyperkalemia in other conditions, not the primary treatment for Addisonian crisis.
B (Administer IV hydrocortisone): Correct. Hydrocortisone addresses the critical lack of cortisol, improving blood pressure and electrolyte balance.
C (Restrict sodium intake): Incorrect. Sodium should be supplemented because aldosterone deficiency leads to sodium loss.
D (Monitor for signs of infection): Incorrect. While important, it is not the immediate priority in a crisis.


