Fluid, electrolyte, and acid-base balance is a fundamental concept in critical care and a key topic for the NCLEX-RN® exam. It involves the regulation of body fluids, electrolytes, and pH to maintain homeostasis and support vital functions. Imbalances can arise from conditions like dehydration, kidney failure, or metabolic disturbances, posing serious health risks. Nurses play a pivotal role in assessing, monitoring, and managing these imbalances through interventions like fluid replacement, electrolyte correction, and acid-base adjustments to ensure patient safety and stability.
Learning Objectives
In studying “Critical Care: Fluid, Electrolyte, Acid-Base Balance” for the NCLEX-RN® Exam, you should learn to understand the mechanisms of fluid regulation, electrolyte homeostasis, and acid-base balance in maintaining physiological stability. Analyze clinical presentations of imbalances like dehydration, hyperkalemia, and acidosis, and evaluate the interventions used to correct these disturbances. Explore the role of diagnostic tools like arterial blood gases (ABGs) and lab tests in assessing patient status. Additionally, apply critical thinking to prioritize nursing actions and interpret fluid, electrolyte, and acid-base balance scenarios in NCLEX-style practice questions, emphasizing patient safety and effective care management strategies.
Fluid Balance
Fluid balance in critical care involves maintaining the equilibrium of fluids within the body to support organ function, optimize hemodynamics, and prevent complications such as fluid overload or dehydration.
Key Concepts
Body Compartments:
- Intracellular Fluid (ICF): Constitutes about 66% (2/3) of the total body water. It is primarily located within cells and plays a crucial role in cellular functions such as metabolism and nutrient transport.
- Extracellular Fluid (ECF): Comprises about 33% (1/3) of the total body water and is further divided into:
- Interstitial Fluid: Surrounds the cells and acts as a medium for the exchange of nutrients and waste products.
- Plasma: The fluid portion of blood, critical for maintaining blood pressure and transporting electrolytes and proteins.
Mechanisms of Regulation:
- Osmosis: Movement of water from areas of low solute concentration to high solute concentration to achieve equilibrium.
- Diffusion: Passive movement of solutes from areas of high concentration to low concentration.
- Active Transport: Uses energy (ATP) to move solutes against a concentration gradient, such as the sodium-potassium pump.
- Hormonal Regulation:
- Antidiuretic Hormone (ADH): Increases water reabsorption in the kidneys, reducing urine output during dehydration.
- Aldosterone: Promotes sodium reabsorption and potassium excretion in the kidneys, aiding in fluid retention.
- Atrial Natriuretic Peptide (ANP): Opposes aldosterone, promoting sodium and water excretion to reduce blood volume and pressure.
Assessment:
- Fluid Overload:
- Physical signs: Peripheral edema, pulmonary crackles, weight gain.
- Vital signs: Elevated blood pressure, bounding pulses.
- Dehydration:
- Physical signs: Sunken eyes, dry mucous membranes, decreased skin turgor.
- Vital signs: Hypotension, tachycardia.
Electrolyte Imbalances
Sodium (Na⁺):
- Hyponatremia:
- Causes: SIADH, renal failure, overuse of diuretics, excessive water intake.
- Symptoms: Headache, lethargy, confusion, seizures, coma.
- Treatment: Fluid restriction, hypertonic saline (3% NS) in severe cases.
- Hypernatremia:
- Causes: Excessive water loss (e.g., diarrhea, fever), hyperaldosteronism.
- Symptoms: Dry mucous membranes, thirst, irritability, seizures.
- Treatment: Oral or IV hypotonic fluids (0.45% NS), treat underlying cause.
Potassium (K⁺):
- Hypokalemia:
- Causes: Use of loop diuretics, GI losses (vomiting, diarrhea), metabolic alkalosis.
- Symptoms: Muscle cramps, weakness, ileus, ECG changes (flat T waves, U waves).
- Treatment: Oral or IV potassium replacement (max infusion rate: 10-20 mEq/hr).
- Hyperkalemia:
- Causes: Renal failure, potassium-sparing diuretics, acidosis.
- Symptoms: Muscle weakness, paresthesia, ECG changes (peaked T waves, widened QRS).
- Treatment: Calcium gluconate (stabilizes cardiac membrane), insulin with dextrose, sodium polystyrene sulfonate (Kayexalate).
Calcium (Ca²⁺):
- Hypocalcemia:
- Causes: Hypoparathyroidism, vitamin D deficiency, acute pancreatitis.
- Symptoms: Tetany, Chvostek’s and Trousseau’s signs, seizures.
- Treatment: Calcium gluconate or calcium chloride IV, vitamin D supplementation.
- Hypercalcemia:
- Causes: Hyperparathyroidism, malignancy, prolonged immobilization.
- Symptoms: Fatigue, kidney stones, cardiac arrhythmias.
- Treatment: IV fluids, bisphosphonates, calcitonin.
Magnesium (Mg²⁺):
- Hypomagnesemia:
- Causes: Alcoholism, malabsorption, diuretics.
- Symptoms: Tremors, hyperreflexia, ECG changes (prolonged QT).
- Treatment: Oral or IV magnesium sulfate.
- Hypermagnesemia:
- Causes: Renal failure, excessive magnesium intake (e.g., antacids, laxatives).
- Symptoms: Hyporeflexia, bradycardia, respiratory depression.
- Treatment: IV calcium gluconate, loop diuretics, dialysis if severe.
Acid-Base Balance
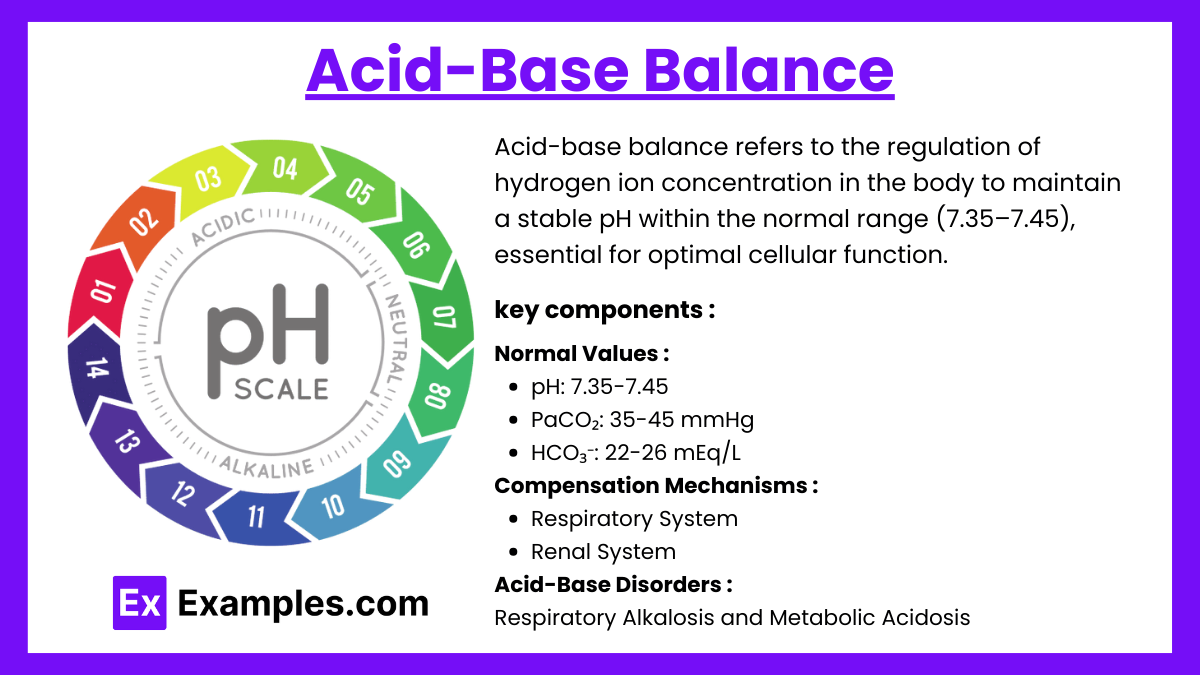
Acid-base balance refers to the regulation of hydrogen ion concentration in the body to maintain a stable pH within the normal range (7.35–7.45), essential for optimal cellular function. It involves interactions between respiratory, renal, and buffering systems.
Normal Values:
- pH: 7.35-7.45 (indicates acid-base status).
- PaCO₂: 35-45 mmHg (respiratory component).
- HCO₃⁻: 22-26 mEq/L (metabolic component).
Compensation Mechanisms:
- Respiratory System: Rapid regulation by adjusting CO₂ levels through changes in respiratory rate and depth.
- Renal System: Slower, more effective regulation by excreting hydrogen ions (H⁺) and reabsorbing bicarbonate (HCO₃⁻).
Acid-Base Disorders:
- Respiratory Acidosis:
- Causes: Hypoventilation (e.g., COPD, drug overdose).
- Symptoms: Confusion, headache, dyspnea, hyperkalemia.
- Compensation: Kidneys increase HCO₃⁻ reabsorption.
- Respiratory Alkalosis:
- Causes: Hyperventilation due to:
- Anxiety, panic attacks.
- High-altitude exposure.
- Fever, sepsis, or hypoxemia.
- Symptoms:
- Dizziness, lightheadedness.
- Tingling (paresthesia), muscle cramps.
- Hypocalcemia (calcium binds to albumin in alkalosis).
- Compensation: Kidneys excrete bicarbonate (HCO₃⁻) and retain H⁺.
- Causes: Hyperventilation due to:
- Metabolic Acidosis:
- Causes: Excess acid production or bicarbonate loss:
- Diabetic ketoacidosis (DKA).
- Lactic acidosis (sepsis, shock).
- Renal failure.
- Diarrhea (bicarbonate loss).
- Symptoms:
- Kussmaul respirations (deep, labored breathing).
- Lethargy, confusion.
- Hyperkalemia, hypotension.
- Compensation: Lungs increase ventilation to lower PaCO₂.
Examples
Example 1: Managing Hyperkalemia in a Renal Failure Patient
A client with chronic kidney disease presents with serum potassium of 6.8 mEq/L, muscle weakness, and peaked T waves on the ECG. As a critical care nurse, you anticipate administering calcium gluconate to stabilize the cardiac membrane, followed by insulin and dextrose to shift potassium intracellularly. Sodium polystyrene sulfonate (Kayexalate) may be used to promote potassium excretion. The NCLEX-RN® question might ask about prioritizing interventions or recognizing ECG changes related to hyperkalemia.
Example 2: Treating Fluid Overload in Heart Failure
A client with congestive heart failure (CHF) exhibits signs of fluid overload, including dyspnea, crackles in the lungs, peripheral edema, and elevated blood pressure. The nurse administers IV furosemide (Lasix) to promote diuresis and monitors intake/output and daily weights. The NCLEX-RN® might test knowledge of recognizing fluid overload symptoms or safe administration and monitoring of diuretics.
Example 3: Recognizing Metabolic Acidosis in Diabetic Ketoacidosis (DKA)
A client with type 1 diabetes is admitted with blood glucose of 550 mg/dL, pH of 7.25, and bicarbonate of 15 mEq/L. Symptoms include Kussmaul respirations, confusion, and fruity-smelling breath. The priority intervention involves IV insulin to correct hyperglycemia and fluids to address dehydration. Questions could focus on identifying metabolic acidosis from ABG values or prioritizing interventions to correct the underlying cause.
Example 4: Managing Hyponatremia in Syndrome of Inappropriate Antidiuretic Hormone (SIADH)
A client diagnosed with SIADH presents with serum sodium of 125 mEq/L, confusion, and lethargy. The nurse restricts fluids, administers hypertonic saline (3% NS) cautiously to prevent rapid correction, and monitors for symptoms of cerebral edema or fluid shifts. NCLEX-RN® questions may assess understanding of the causes of hyponatremia and appropriate management strategies to prevent complications such as central pontine myelinolysis.
Example 5: Addressing Respiratory Alkalosis in a Client with Anxiety
A client experiencing a panic attack exhibits rapid, shallow breathing, tingling in the fingers, and a feeling of dizziness. ABG analysis reveals pH of 7.48 and PaCO₂ of 30 mmHg. The nurse coaches the client in slow, deep breathing techniques or uses a paper bag to help retain CO₂. NCLEX-RN® questions may test your ability to recognize respiratory alkalosis and apply non-pharmacological interventions to restore acid-base balance.
Practice Questions
Question 1
A patient with severe vomiting for the past 48 hours presents with confusion, muscle cramps, and a potassium level of 2.8 mEq/L. What is the nurse’s priority intervention?
A. Administer 3% sodium chloride intravenously.
B. Begin an IV infusion of potassium chloride (KCl) as prescribed.
C. Administer calcium gluconate to stabilize the heart.
D. Encourage oral fluid intake.
Answer: B. Begin an IV infusion of potassium chloride (KCl) as prescribed.
Explanation:
- The patient exhibits symptoms of hypokalemia, which include confusion, muscle weakness or cramps, and arrhythmias.
- Potassium chloride (KCl) is the appropriate treatment to correct hypokalemia. IV potassium is administered cautiously, usually diluted and infused slowly to avoid complications like cardiac arrhythmias or vein irritation.
- Option A: Hypertonic sodium chloride is used for severe hyponatremia, not potassium imbalances.
- Option C: Calcium gluconate is indicated for hyperkalemia to protect the heart but is not appropriate for hypokalemia.
- Option D: Oral fluids alone will not correct hypokalemia caused by severe vomiting.
Question 2
A patient with chronic obstructive pulmonary disease (COPD) has an arterial blood gas (ABG) reading: pH 7.30, PaCO₂ 55 mmHg, HCO₃⁻ 28 mEq/L. How should the nurse interpret these findings?
A. Respiratory acidosis with partial compensation.
B. Metabolic acidosis with no compensation.
C. Metabolic alkalosis with full compensation.
D. Respiratory alkalosis with partial compensation.
Answer: A. Respiratory acidosis with partial compensation.
Explanation:
- ABG Interpretation:
- pH < 7.35 = Acidosis.
- PaCO₂ > 45 mmHg = Indicates respiratory origin.
- HCO₃⁻ > 26 mEq/L = Partial metabolic compensation.
- The patient has respiratory acidosis due to COPD (impaired gas exchange leads to CO₂ retention). The kidneys attempt to compensate by retaining HCO₃⁻, but the pH has not normalized, indicating partial compensation.
- Option B: Incorrect because the primary issue is respiratory, not metabolic.
- Option C: Incorrect as alkalosis is not present in these ABG values.
- Option D: Incorrect because PaCO₂ indicates acidosis, not alkalosis.
Question 3
A patient receiving a loop diuretic for heart failure develops muscle weakness, lethargy, and a serum sodium level of 125 mEq/L. Which additional finding is most concerning?
A. A urine specific gravity of 1.010.
B. A serum calcium level of 8.8 mg/dL.
C. A serum potassium level of 2.9 mEq/L.
D. Blood pressure of 110/70 mmHg.
Answer: C. A serum potassium level of 2.9 mEq/L.
Explanation:
- Hypokalemia is a common side effect of loop diuretics and poses significant risks, such as cardiac arrhythmias.
- Serum potassium < 3.5 mEq/L requires immediate intervention to prevent life-threatening complications.
- Option A: A urine specific gravity of 1.010 reflects dilute urine, which is expected with loop diuretics and hyponatremia.
- Option B: A serum calcium level of 8.8 mg/dL is on the lower end of normal but less concerning than hypokalemia.
- Option D: Blood pressure of 110/70 mmHg is normal and not a priority concern.


