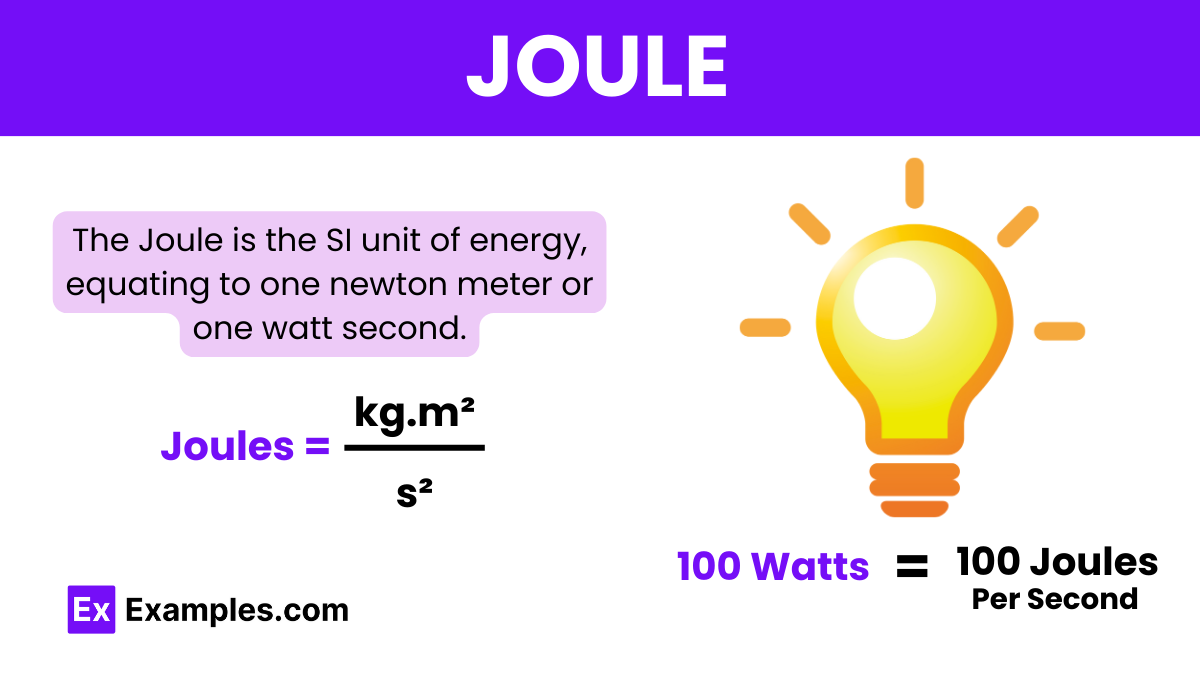
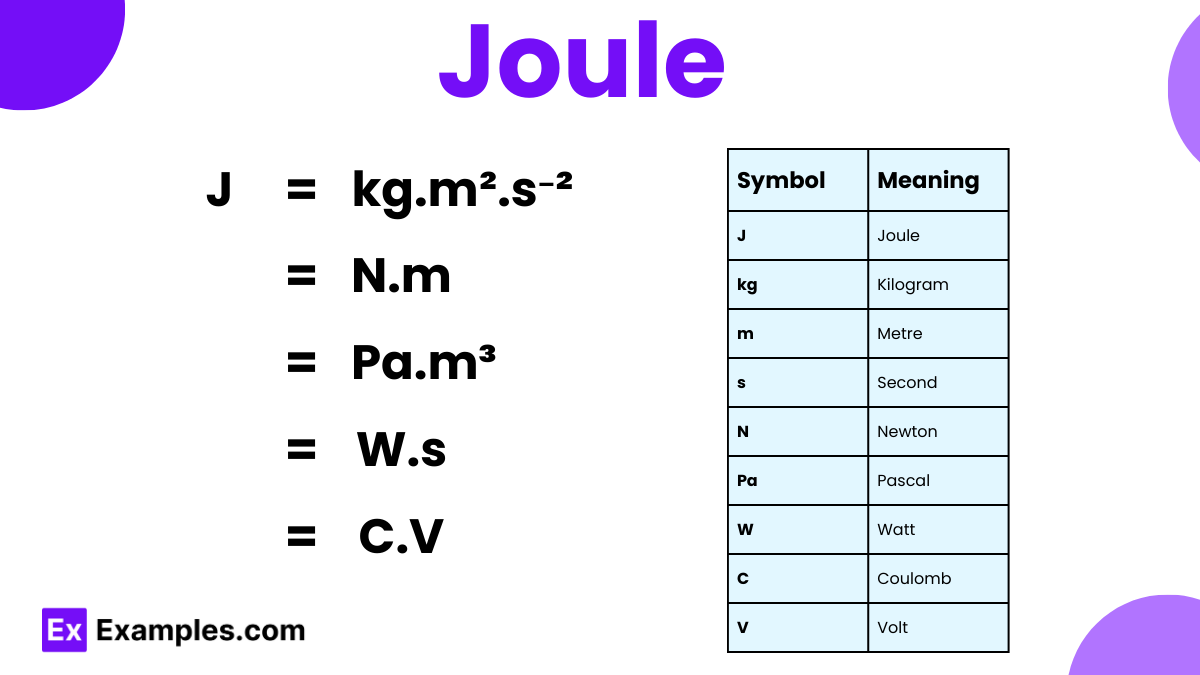
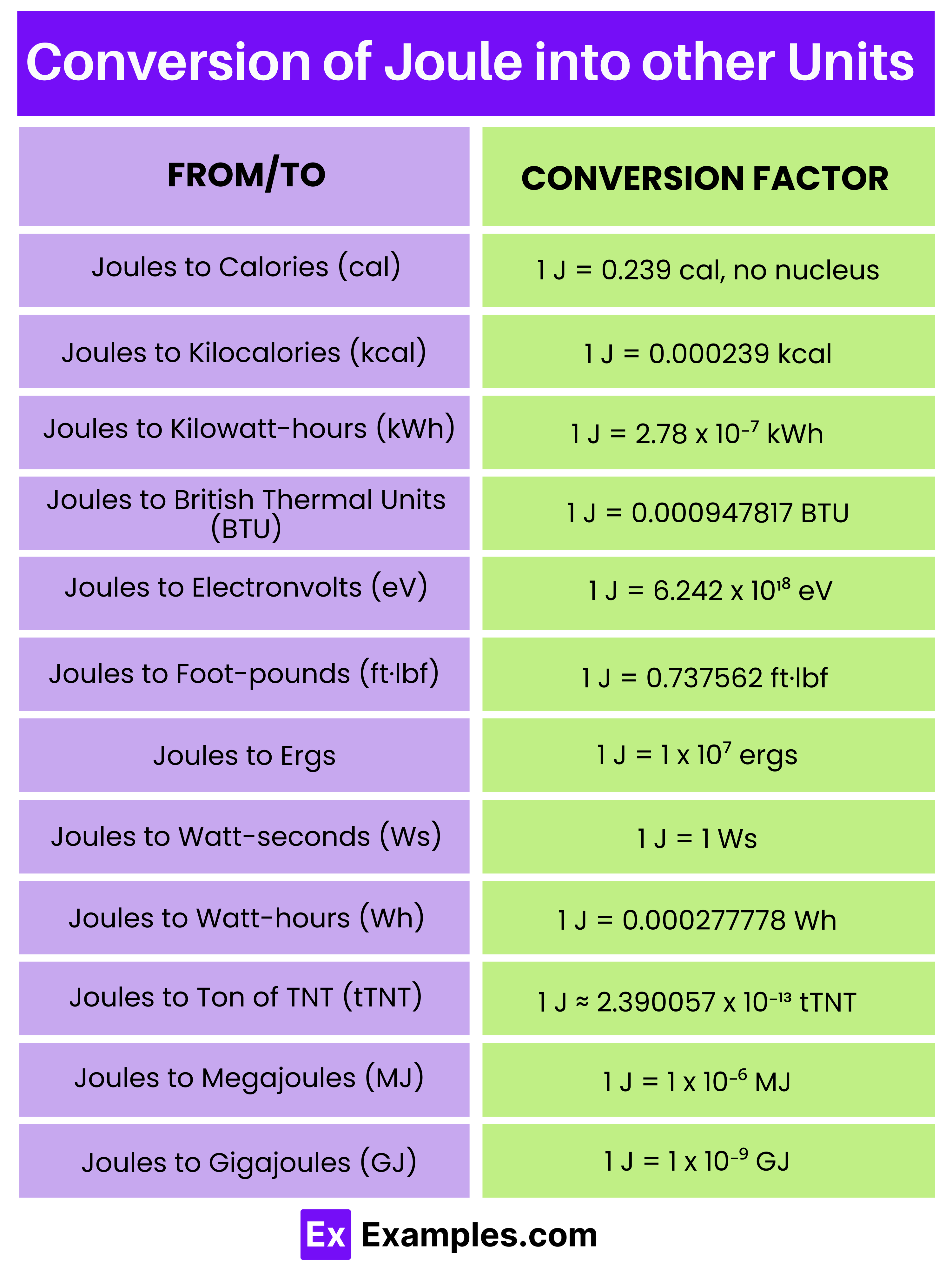
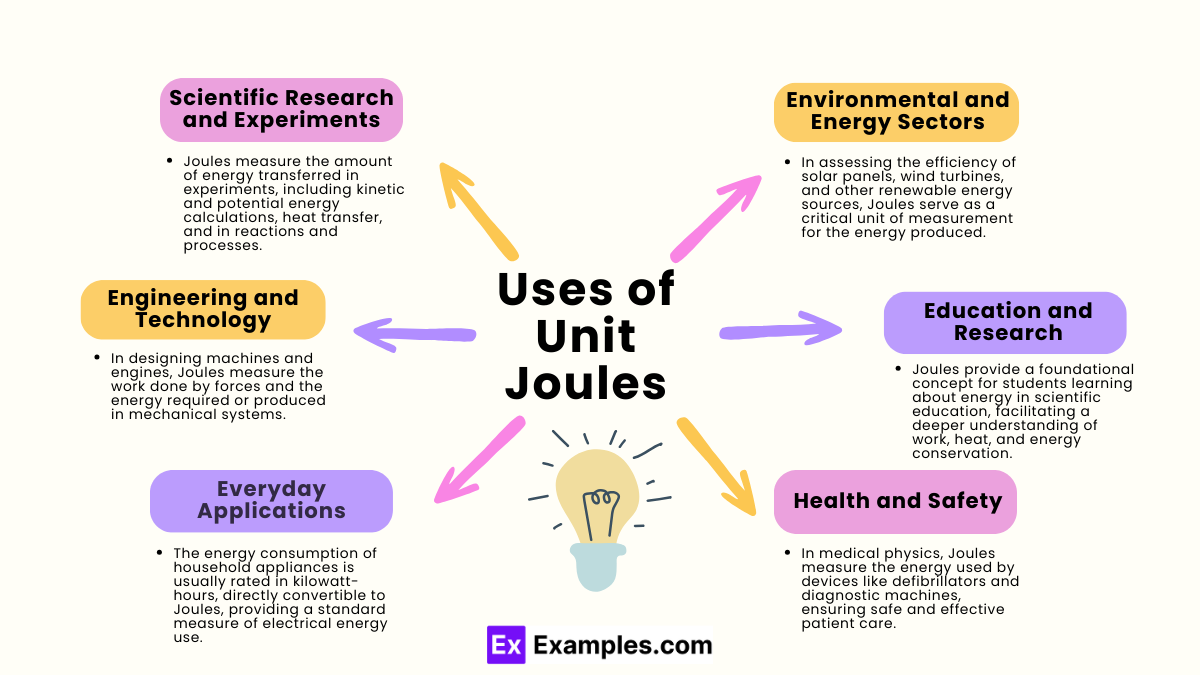
What is the definition of a joule?
The energy required to raise the temperature of 1 gram of water by 1 degree Celsius
The energy transferred when a force of 1 newton moves an object 1 meter
The power required to do 1 joule of work in 1 second
The energy stored in 1 kilogram of mass